The Glycemia Reduction Approaches in Type 2 Diabetes – A comparative effectiveness (GRADE) Study[1] was a study to assess the effectiveness of various add-on therapies to metformin in patients with recent onset diabetes mellitus
- Duration of the study : May 2013 to April 2021
- Location : United States (36 academic centers)
- Sponsor : NIDDK (National Institutes of Health)

Rationale and Clinical Equipoise
For most patients with recently diagnosed type 2 diabetes mellitus (less than ten years) with limited diabetes-specific complications, a glycated hemoglobin level of 7.0% or less (<53 mmol per mole) has been widely adopted in various consensus guidelines as a clinically reasonable treatment goal. Furthermore, metformin (an insulin sensitizer) has been the preferred initial choice of monotherapy for type 2 diabetes mellitus.
At the time of initiation of this study (around 2013), there was significant uncertainty regarding the ideal second-line treatment (add-on therapy) option for type 2 diabetes mellitus. It is worth noting that therapeutic options available during study initiation included metformin, pioglitazone, DPP4-inhibitors, GLP-1 agonists, and insulin. SGLT-1 inhibitors were, at the time, still undergoing regulatory review and approval. Due to cardiovascular and malignancy safety concerns, a pioglitazone arm of the study was excluded.
Study Design
- A multicenter, parallel-group, comparative-effectiveness study
- The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health sponsored the study.
- Stratified randomization across 36 clinical centers in the United States.
- Patients and clinical staff were unblinded to treatment assignments. Laboratory staff and adjudicating committee members were not aware of group assignments.
- An initial run-in period of 6-14 weeks was required to allow assessment of metformin tolerance and up-titration to a maximum dose of 2000mg per day.
- Participants were expected to have an A1C of 6.8-8.5% at the end of the run-in period before study group assignments (randomization).
Study Eligibility Criteria
- Eligible subjects ≥ 30 years (≥ 20 for American Indians) of age.
- Duration of type 2 diabetes mellitus < 10 years
- On metformin monotherapy
- Extensive exclusion criteria [2] included suspected autoimmune diabetes, monogenic diabetes, pancreatogenic diabetes, history of congestive heart failure (NYHA Class III), history of cancer, pancreatitis, previous organ transplant, major cardiovascular event in the previous year, and symptomatic hyperglycemia to mention a few (review others here)
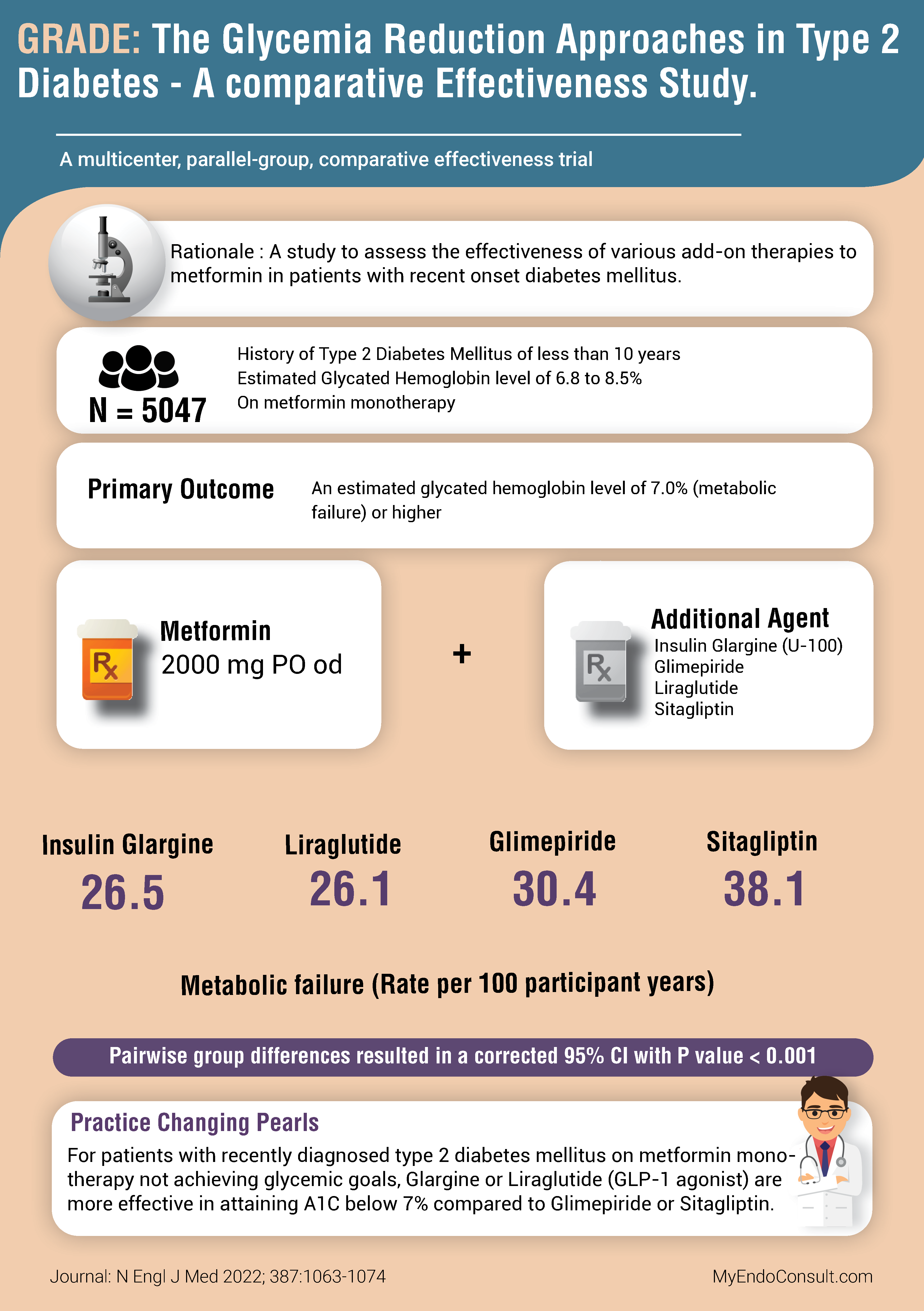
Primary Outcome
The time to an A1C value ≥ 7% on dual therapy with metformin (plus an additional agent). Assessed quarterly over a 4-7 year period. An A1C above this threshold was defined as metabolic (treatment) failure.
The rate per 100 participant years was 26.5, 26.1, 30.4, and 38.1 for insulin glargine, liraglutide, glimepiride, and sitagliptin, respectively. Pairwise group differences resulted in a 95% confidence interval with a p-value <0.001.
Discussion
There has been a recent paradigm shift in therapeutic algorithms for managing type 2 diabetes. Notably, there has been a departure from the previous stepped approach to treatment which involved metformin as the initial therapeutic choice. After the FDA’s requirement for all new diabetes drugs to have demonstrable cardiovascular safety data, more recent diabetes medications (GLP-1 agonists and SGLT-2 inhibitors) have shown significant efficacy in improving both ASCVD and renal outcomes. In line with this, most current guidelines recommend using specific agents as either monotherapy or combination therapy in patients deemed at high risk for adverse cardiovascular outcomes. The results of the GRADE study can, however, guide the approach to treating low-risk and relatively healthy patients with recent onset diabetes mellitus.
Bottom line: The application of the study results should be guided by patient-stated goals, cost, convenience of administration, hypoglycemia risk, risk of weight gain, and other metabolic complications. GLP-1 agonists and insulin showed greater efficacy in a sustained reduction in glycated hemoglobin levels below 7%. It is worth noting that quality of life issues, cost, long-term acceptance of injectable agents, etc., were not assessed in this study. These factors could undoubtedly impact treatment goals outside a clinical trial setting.
Critical Appraisal
Was there aggressive up-titration of insulin secretagogues/insulin in this study?
Although investigators involved in data analysis were blinded to treatment assignments, treating physicians were unblinded to group assignments. This could have introduced bias regarding a clinician’s approach to attaining glycemic goals. For example, titration of sulfonylurea or basal insulin in patients with fasting hypoglycemia can undoubtedly be influenced by a clinician’s knowledge of a patient’s group assignment. It is interesting to note that although the risk of hypoglycemia was as expected, high in the glimepiride and basal insulin groups compared to liraglutide and sitagliptin groups, patients in the former two groups had a relatively stable weight throughout the study. It is theoretically possible that less aggressive up-titration in the dose of sulfonylurea therapy among patients with hypoglycemia could have accounted for the lower efficacy of this treatment arm.
What happened to patients with newly diagnosed ASCVD?
During the study, new cardiovascular outcome data became available regarding the therapeutic benefits of selected GLP-1 agonists and SGLT-2 inhibitors in type 2 diabetes mellitus. The authors did not report the ethical implications of this recent development on treatment decisions during the study. For example, what would have happened to a 45-year-old patient with a new-onset acute myocardial infarction requiring stent placement? If this patient was in the sulfonylurea arm of the study, what next steps would have been taken?
Practice-Changing Pearls (Conclusion)
For patients with recently diagnosed type 2 diabetes mellitus on metformin monotherapy not achieving glycemic goals, Glargine or Liraglutide (GLP-1 agonist) are more effective in attaining A1C below 7% compared to Glimepiride or Sitagliptin.
References
- (2022) Glycemia Reduction in Type 2 Diabetes — Glycemic Outcomes. N Engl J Med 387:1063–1074
- GRADE Study Group (2022) Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study. clinicaltrials.gov
Kindly Let Us Know If This Was helpful? Thank You!


