The Thyroid Gland: Structure And Histology
Embryologically, the thyroid gland originates from an epithelial invagination at the base of the fetal tongue. It comprises two sizable lobes, typically weighing between 15–20 g, positioned on either side of the trachea and just below the larynx or voice box. These lobes connect via a narrow strand of thyroid tissue, termed the isthmus. Occasionally, a small pyramidal tissue extension emerges upward from the isthmus. The thyroid arteries deliver a profuse blood supply to the gland, which also benefits from a rich innervation from the autonomic nervous system. However, these nerves appear to have a minimal role in controlling thyroid blood flow and function. A fibrous connective tissue capsule completely envelopes the gland.
In histological terms, the thyroid tissue consists of numerous spherical follicles. Each follicle is composed of a rim of simple cuboidal epithelial cells encircling a mass of colloidal storage protein named thyroglobulin. Depending on the functional state of the gland, the height of this cuboidal epithelium can fluctuate. When the gland is relatively inactive, the colloidal mass enlarges and the surrounding follicular cells flatten. In contrast, under hyperactive conditions, the follicular lumen shrinks and the endocrine cells adopt a columnar appearance. Separating the follicles into various functional lobules are fingers of loose connective tissue that contain blood vessels, lymphatics, and nerve fibres.
Parafollicular C cells, larger separate groups of epithelial cells situated between the thyroid follicles, are responsible for the secretion of the peptide hormone calcitonin, which plays a role in the regulation of calcium metabolism. While these C-cells may also inhabit the follicular epithelium, they never border the follicular lumen
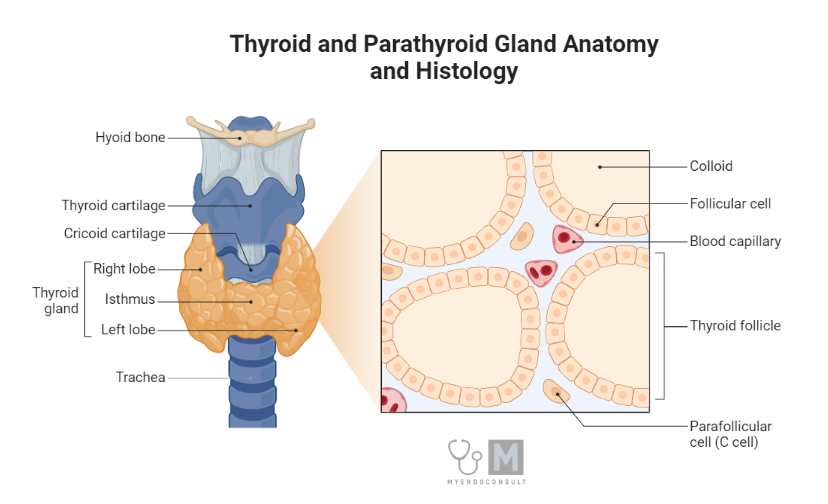
Anatomy and histology of the thyroid gland
Types of thyroid hormones
Thyroid hormones are important regulators of various physiological processes, such as cellular growth and metabolism. The thyroid gland present in the anterior neck is responsible for the synthesis and secretion of thyroid hormones: thyroxine (T4) and triiodothyronine (T3).
Firstly, T4 is the predominant thyroid hormone secreted by the thyroid gland, accounting for approximately 90% of endogenous total thyroid hormone production[1]. It should be noted that T4 is, in fact, a prohormone with relatively low biological activity compared to T3[1,2]. Structurally, T4 comprises four iodine atoms bound to a tyrosine-derived backbone. Most thyroxine is bound to carrier proteins, such as thyroxine-binding globulin (TBG), transthyretin, and albumin in circulation, with only a tiny fraction (approximately 0.03%) present as free thyroxine (FT4)[3]. Free T4 is the biologically active form that can enter target cells and exert various intracellular effects.
On the other hand, T3, the biologically active thyroid hormone, accounts for almost 10% of the total endogenous thyroid hormone production[1]. T3 has approximately three to five times greater potency than T4 and is produced within the thyroid gland through peripheral deiodination.
T3 circulates bound to carrier proteins (mainly TBG and albumin), with a small fraction (approximately 0.3%) existing as free triiodothyronine (FT3). Free T3 exerts its effects by binding to its cognate intracellular thyroid hormone receptors (TRs)[3].
Reverse T3 (rT3) is an inactive metabolite of T4, generated by the action of type 3 deiodinase[4,5], which selectively removes an iodine atom from the inner ring of T4[5]. Although rT3 shares structural similarity with T3, it does not bind to TRs with high affinity and, therefore, does not exhibit any significant biological activity. rT3 is a by-product of thyroid hormone inactivation and is clinically useful as an important marker of the sick euthyroid syndrome[6].
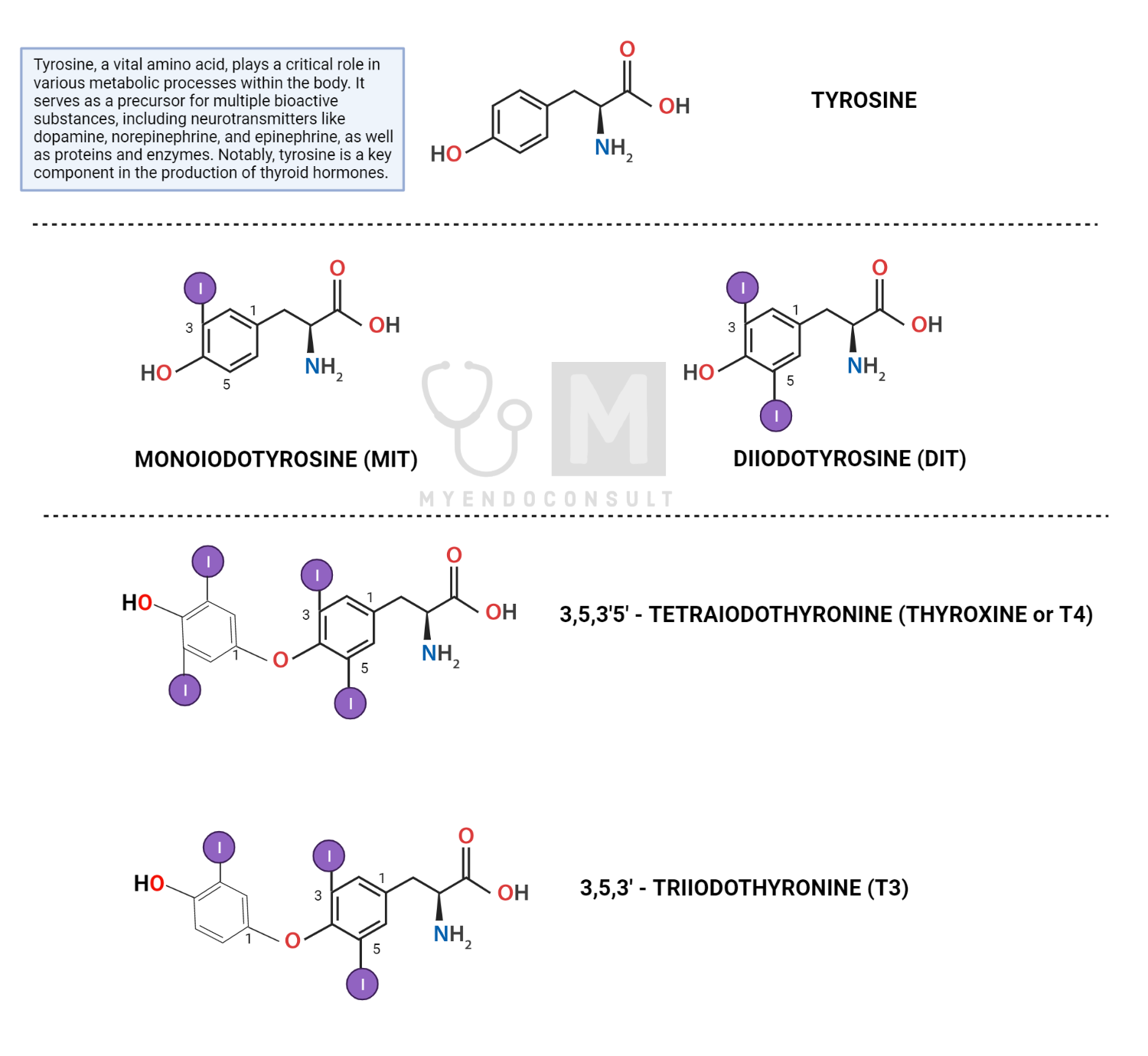
Structure of thyroid hormones, showing the tyrosine backbone
Mechanism of thyroid hormone synthesis
Thyroid hormone synthesis is a highly regulated process that occurs in the thyroid gland, specifically within the thyroid follicular cells. Sodium-iodide symporters (NIS) actively transport iodide ions from the bloodstream into thyroid follicular cells against a sodium electrochemical gradient, which is maintained by the sodium-potassium pump (Na+/K+-ATPase). This results in an elevated intra-follicular iodide concentration compared to the plasma.
Iodide ions are then transported to the apical membrane of the thyroid follicular cells by the action of pendrin, an anion exchanger protein. At the apical membrane of the thyroid follicular cell, the thyroid peroxidase (TPO) enzyme, in the presence of hydrogen peroxide (H2O2), catalyzes the oxidation of iodide to form reactive iodine species[7]. Subsequently, thyroglobulin (Tg), a large glycoprotein containing multiple tyrosine residues, is synthesized in the rough endoplasmic reticulum of thyroid follicular cells. After posttranslational modifications in the Golgi apparatus, Tg is secreted into the follicular lumen (colloid) through exocytosis.
Next, reactive iodine species generated at the apical membrane react with the tyrosine residues present on Tg, a process known as organification. This results in the formation of monoiodotyrosine (MIT) and diiodotyrosine (DIT), iodinated tyrosine derivatives[8].
TPO enzyme also catalyzes the coupling of iodotyrosines (MIT and DIT). The coupling of one DIT and one MIT yields triiodothyronine (T3), while the coupling of two DIT molecules produces tetraiodothyronine (T4, also known as thyroxine).
Colloid-containing iodinated Tg is internalized by the thyroid follicular cells through endocytosis. The colloid-laden endosomes fuse with lysosomes containing proteolytic enzymes, which cleave Tg and release T3 and T4[9].
T3 and T4 are transported across the basolateral membrane of thyroid follicular cells into the perifollicular capillaries. Most T3 and T4 molecules circulate bound to carrier proteins, such as thyroxine-binding globulin (TBG), transthyretin, and albumin[10].
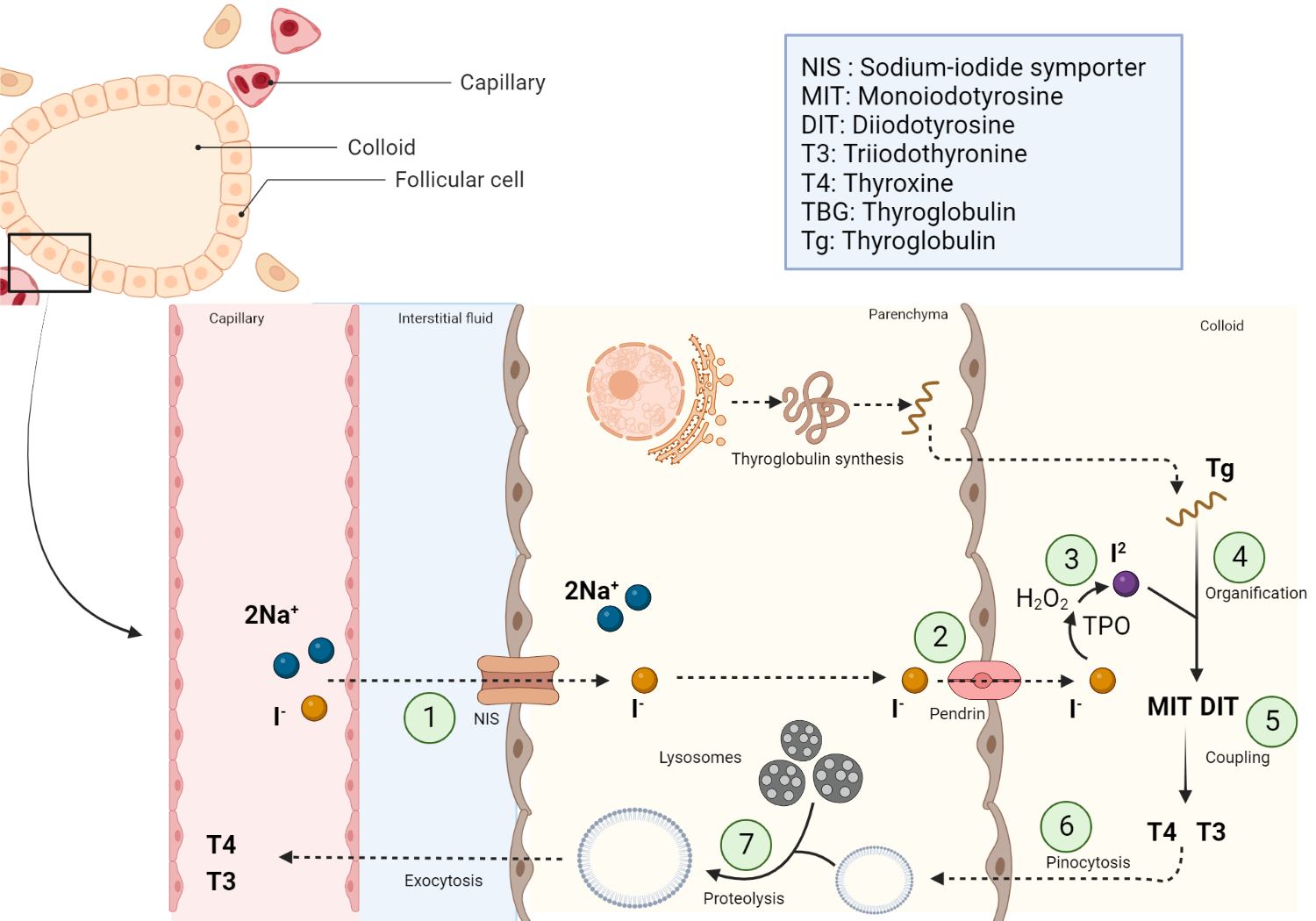
Schematic representation of thyroid hormone synthesis
Summary of the steps involved in the synthesis of thyroid hormone. NIS = Sodium iodide symporter, T4 = tetraiodothyronine, T3 = triiodothyronine, MIT = monoiodotyrosine (MIT), DIT = diiodotyrosine
Transport of thyroid hormones in plasma
As was previously mentioned, thyroid hormones, specifically thyroxine (T4) and triiodothyronine (T3) are bound to carrier proteins in the general circulation. These carrier proteins ferry thyroid hormones throughout the body, thus regulating their availability to target tissues. Indeed, more than 95% of serum thyroid hormones are bound to three major carrier proteins, leaving a small, unbound fraction[2]. Thyroid-binding globulin (TBG) is the primary and most abundant carrier protein, which binds approximately 70% of T4 and 80% of T3[11].
Transthyretin, or thyroxine-binding prealbumin (TBPA), binds about 10-15% of T4 and a negligible proportion of T3 [12]. Finally, albumin, the most abundant protein in human serum, has the lowest affinity for thyroid hormone among all carrier proteins. Albumin binds approximately 15-20% of T4 and 5-10% of T3[13]. Although albumin has a relatively lower affinity for thyroid hormones than TBG and transthyretin, its high concentration in the bloodstream means it plays a significant role in the transport of thyroid hormones.
Only a tiny fraction of T4 (approximately 0.03%) and T3 (approximately 0.3%) remains unbound or "free" in the serum[14]. The free fraction of total thyroid hormones in circulation is readily available for cell uptake, where it exerts its intracellular effects. In contrast, protein-bound thyroid hormones are considered a readily accessible store of thyroid hormones that are biologically inert[15].
Mechanisms of Thyroid Hormone Metabolism
Deiodination is the most significant pathway that metabolizes thyroid hormones and regulates T3 bioavailability in human tissues. The thyroid gland produces only a small amount of T3, while most of T3 (roughly 80%) in peripheral tissues is produced by enzymatic deiodination via outer ring deiodination (ORD) of T4. Similarly, the metabolite rT3 is produced through inner ring deiodination (IRD) of T4[16,17]. Hence, the metabolism of thyroid hormones by deiodinase enzymes results in either their activation or inactivation.
Importance of Thyroid Hormone in Protein Synthesis
The primary mode of action involves binding of T3 (active thyroid hormone) to thyroid hormone receptors (TRs), which function as nuclear transcription factors[18]. Once bound, the hormone receptor complex interacts with specific DNA sequences known as thyroid hormone response elements (TREs) on target genes. This interaction can either stimulate or repress the transcription of genes, ultimately influencing protein synthesis and degradation[19,20].
In addition to their genomic effects, thyroid hormones can also have nongenomic actions on target cells. These effects are typically faster and do not involve direct interaction with DNA. An example of a non-genomic action of thyroid hormone is its role in stimulating amino acid transport into target cells. This process may involve the interaction of thyroid hormones with membrane-bound proteins or other components of the cell membrane, which influence the transport and availability of amino acids for protein synthesis[21].
Regulation of metabolism
T3 crosses the plasma membrane of the targeted cells and binds to mitochondrial receptors, increasing adenosine triphosphate (ATP) production through oxidative phosphorylation. Furthermore, T3 in the nucleus activates genes involved in the transcription of various proteins involved in energy production. This effect of T3 is often called the ‘calorigenic effect of thyroid hormones”[22].
Furthermore, thyroid hormones play a crucial role in regulating various metabolic processes. Hyperthyroxinemia promotes a hypermetabolic state characterized by increased resting energy expenditure, weight loss, and decreased lipolysis. Conversely, hypothyroxinemia decreases resting energy expenditure, weight gain, and increased lipolysis[23].
Finally, thyroid hormone regulates carbohydrate metabolism through various effects. T3 enhances insulin-dependent glucose uptake into cells, gluconeogenesis, and glycogenolysis, leading to a net increase in plasma glucose[24].
Effects of thyroid hormones on growth and development
Thyroid hormone (TH) is crucial for healthy endochondral ossification, skeletal development, linear growth, bone mass maintenance, and optimal fracture repair[25]. Also, iodothyronine transporters and thyroid hormone receptors (TR) are present in osteoblasts and osteoclasts, which are important cells involved in bone formation and remodeling[26].
Dysregulation of thyroid hormone production has differential effects on bone turnover. For example, hypothyroidism is characterized by a decrease in bone turnover and a prolonged remodeling phase of bone formation, leading to increased bone mass[27]. On the other hand, hyperthyroidism is associated with increased bone turnover and accelerated bone resorption. Consequently, hyperthyroxinemia promotes a decrease in bone mass and increases the risk of osteoporosis[28,29].
Importance of Thyroid Hormones in thermogenesis
Thyroid hormones regulate the basal metabolic rate (BMR) through their effects on thermogenesis, primarily in brown adipose tissue. Brown adipose tissue (BAT), a specialized type of fat tissue, contains many mitochondria, essential in generating energy in the form of ATP (via oxidative phosphorylation). Interestingly, in BAT mitochondria, some of this energy is dissipated as heat[22]. We will explore the role of thyroid hormone in thermogenesis next.
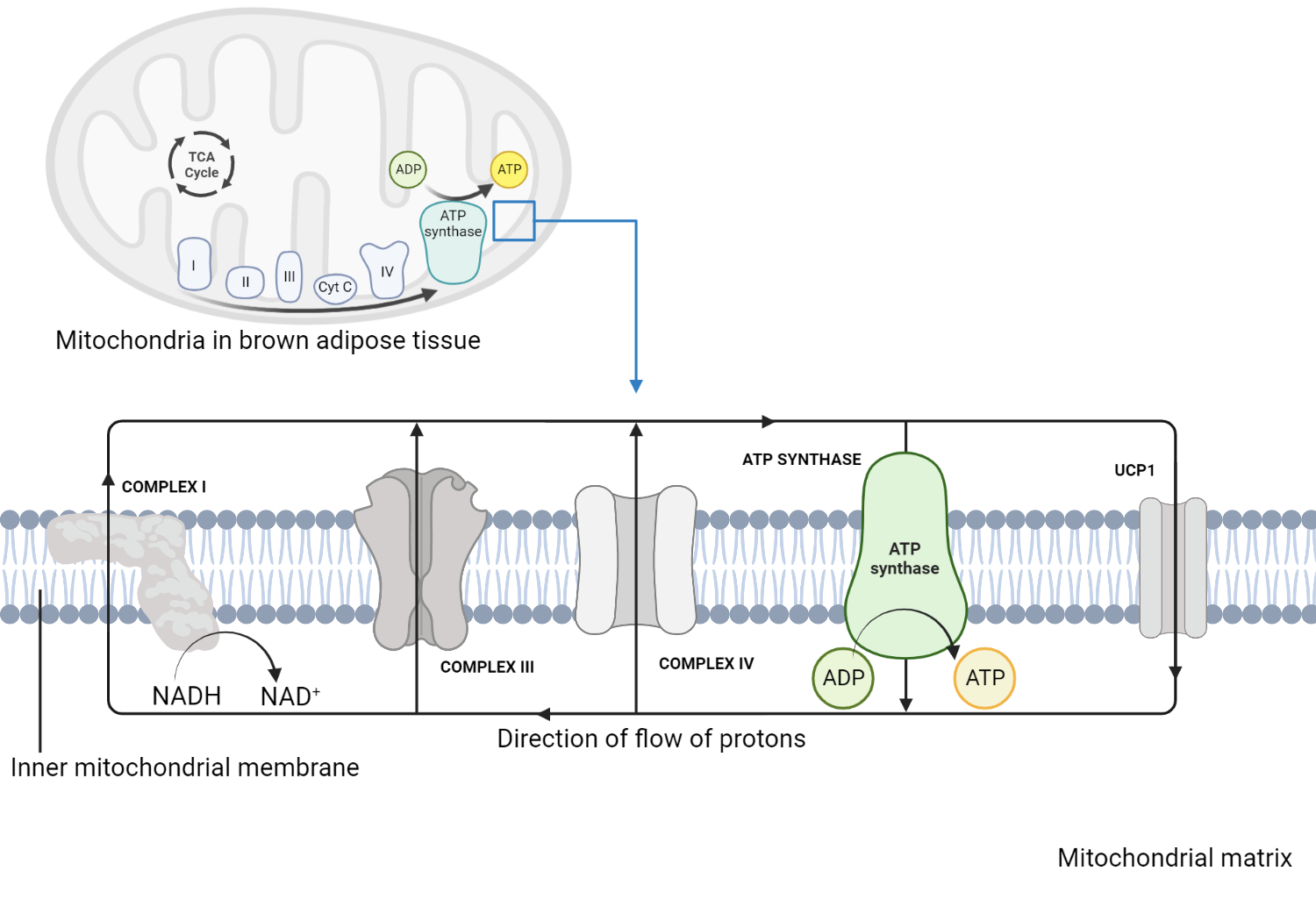
The role of the mitochondria in thermogenesis
Electrons generated as a result of nutrient metabolism (e.g., fatty acids and glucose) are transferred along the electron transport chain (ETC), creating a proton gradient across the inner mitochondrial membrane. Protons accumulate in the intermembrane space before being transferred to the mitochondrial matrix. Indeed instead of driving ATP synthesis, protons flow back into the mitochondrial matrix through a shuttle protein known as Uncoupling Protein 1 (UCP1). The energy of the proton flow is dissipated as heat, which contributes to thermogenesis[30]. T3 promotes the expression of UCP1, increasing the availability of uncoupling proteins in BAT mitochondria. This enhances the proton leak, which augments subsequent heat production. Furthermore, T3 stimulates lipolysis (the breakdown of triglycerides into free fatty acids), which serves as substrates for the production of energy and activators of UCP1[31]. T3 also promotes the generation of new mitochondria in BAT, increasing the capacity of adipose tissue for heat production[32]. Finally, T3 interacts with the sympathetic nervous system, which releases norepinephrine, a hormone that stimulates thermogenesis in BAT[33].
Intracellular Effects of Thyroid Hormone
Once formed, T3 enters the nucleus of target cells and binds to nuclear thyroid hormone receptors (TRs), which are members of the nuclear receptor superfamily. TRs form heterodimers with retinoid X receptors (RXR) and bind to specific DNA sequences called thyroid hormone response elements (TRE) located in the promoter regions of target genes[14,34]. The thyroid hormone receptor that is already bound as a heterodimer with RXR (retinoid X receptors) binds to the specific TH response element sequences (TRE) found in the promoter regions of T3 target genes in the nucleus and controls the expression of these genes in a ligand-dependent manner. On the contrary, unliganded TRs connect with TREs in T3 target genes and control transcriptional repression. In the absence of T3, co-repressor proteins are recruited into the RXR-TR heterodimer and inhibit the expression of the target gene[35,36]. Different types of mRNAs are produced as a result of transcription; these mRNAs then migrate from the nucleus into the cytosol, where they undergo translation to produce proteins[37].
Transcriptional regulation mediated by T3-bound TRs impacts various physiological processes, including basal metabolic rate, protein synthesis, carbohydrate and lipid metabolism, bone growth and development, and nervous system maturation[38,39]. The net effect of levothyroxine treatment is restoring normal thyroid hormone levels and alleviating hypothyroid symptoms.
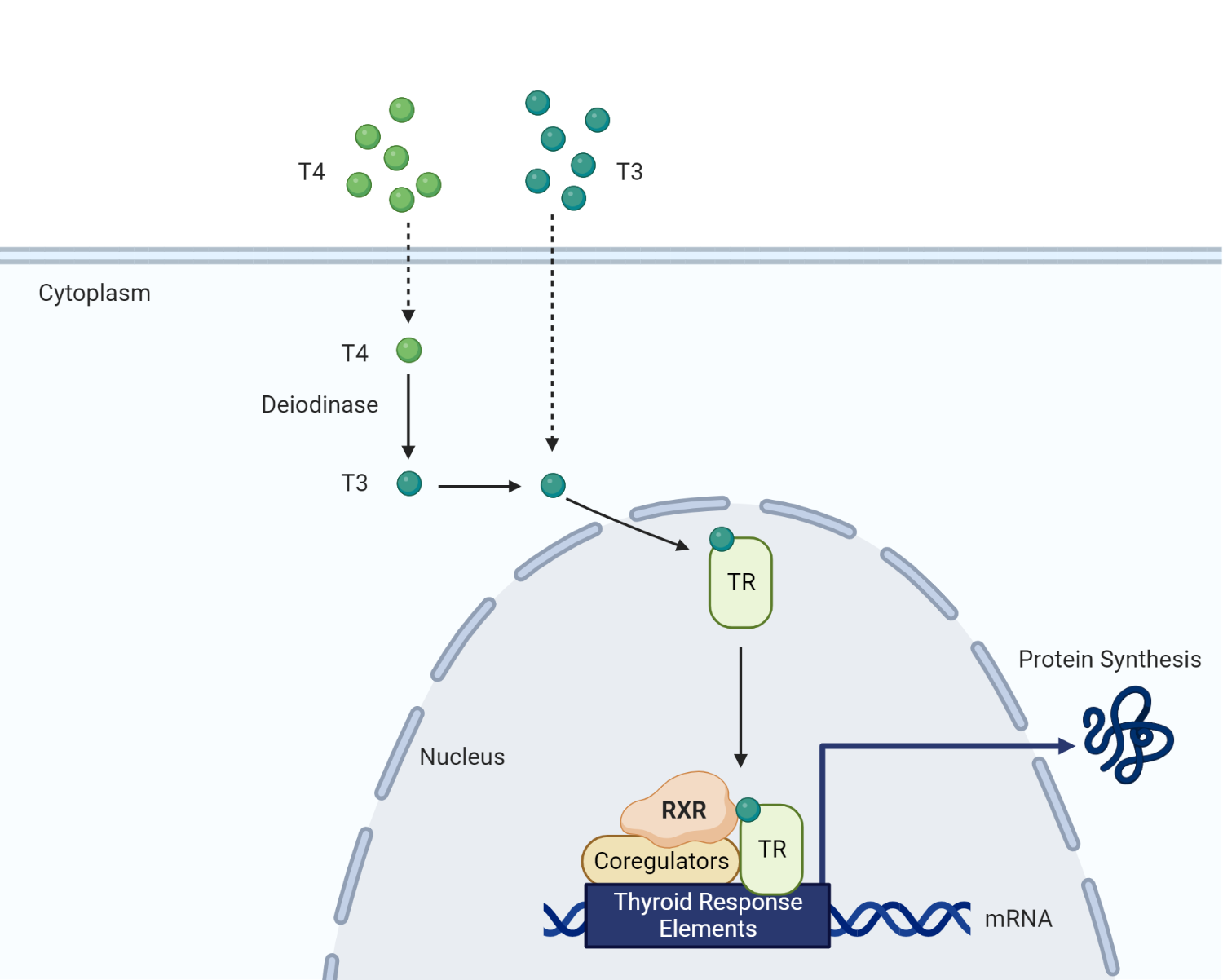
Intracellular effects of thyroid hormone. T3 exerts its intracellular effects by binding to a nuclear thyroid hormone receptor. Along with retinoid X receptors and various coregulators, thyroid hormone facilitates the transcription of regulatory proteins required for metabolism.
Altered thyroid hormone levels: Effects on Physiological Functions
System | Hypothyroidism | Hyperthyroidism |
Basal metabolic rate (BMR) and temperature regulation | Low BMR Decreased body temperature Cold intolerance | High BMR Increased body temperature Heat intolerance |
Carbohydrate metabolism | Decreased glucose metabolism | Increased glucose metabolism. Enhanced glycolysis and gluconeogenesis |
Cardiac | Bradycardia and hypotension | Tachycardia and hypertension |
Gastrointestinal (GI) tract | Decreased GI motility and smooth muscle tone | Increased GI motility |
Hematopoietic | Anemia | - |
Musculoskeletal | Slow relaxation of contracted muscle | Muscle atrophy |
Based on [38]
Mechanism of action of Liothyronine
Liothyronine (LT3), a synthetic form of the endogenous thyroid hormone T3 is absorbed in the gastrointestinal tract and enters the systemic circulation after oral administration. T3 then enters target cells, primarily through thyroid hormone transporters, such as monocarboxylate transporter 8 (MCT8) and organic anion transport polypeptide 1C1 (OATP1C1)[40]. Once inside target cells, liothyronine, the biologically active form of thyroid hormone, binds directly to nuclear thyroid hormone receptors (TRs). The intracellular effects of T3 were reviewed earlier.
Mechanism of action of Tetraiodothyronine
Levothyroxine (LT4) is a synthetic preparation of endogenous thyroid hormone thyroxine (T4), utilized for the treatment of hypothyroidism, irrespective of the underlying cause[41]. In order to exert its physiological effects, levothyroxine is converted by various deiodinases into the biologically active hormone triiodothyronine (T3). As a result, T3 interacts with nuclear thyroid hormone receptors to ultimately modulate gene transcription[42]
Levothyroxine is converted to the active hormone T3 by the action of deiodinase enzymes, primarily type 1 (D1) and type 2 (D2) deiodinases[43,44]. For example, type I deiodinase (D1), which is found predominantly in hepatic and renal tissues, is responsible for the conversion of the prohormone thyroxine (T4) to its biologically active form, triiodothyronine (T3)[14]. Furthermore, D1 converts T4 to reverse T3 (rT3), its inactive metabolite[45].
Type II deiodinase (D2) is present mainly in the brain, pituitary gland, brown adipose tissue, skeletal muscle, and other peripheral tissues. D2 primarily converts T4 to T3 for intracellular use, thus playing a critical role in the local concentration of active thyroid hormone[4]. It is worth noting that negative feedback regulation of the hypothalamic-pituitary-thyroid (HPT) axis depends on T3 generated in the pituitary gland itself[46].
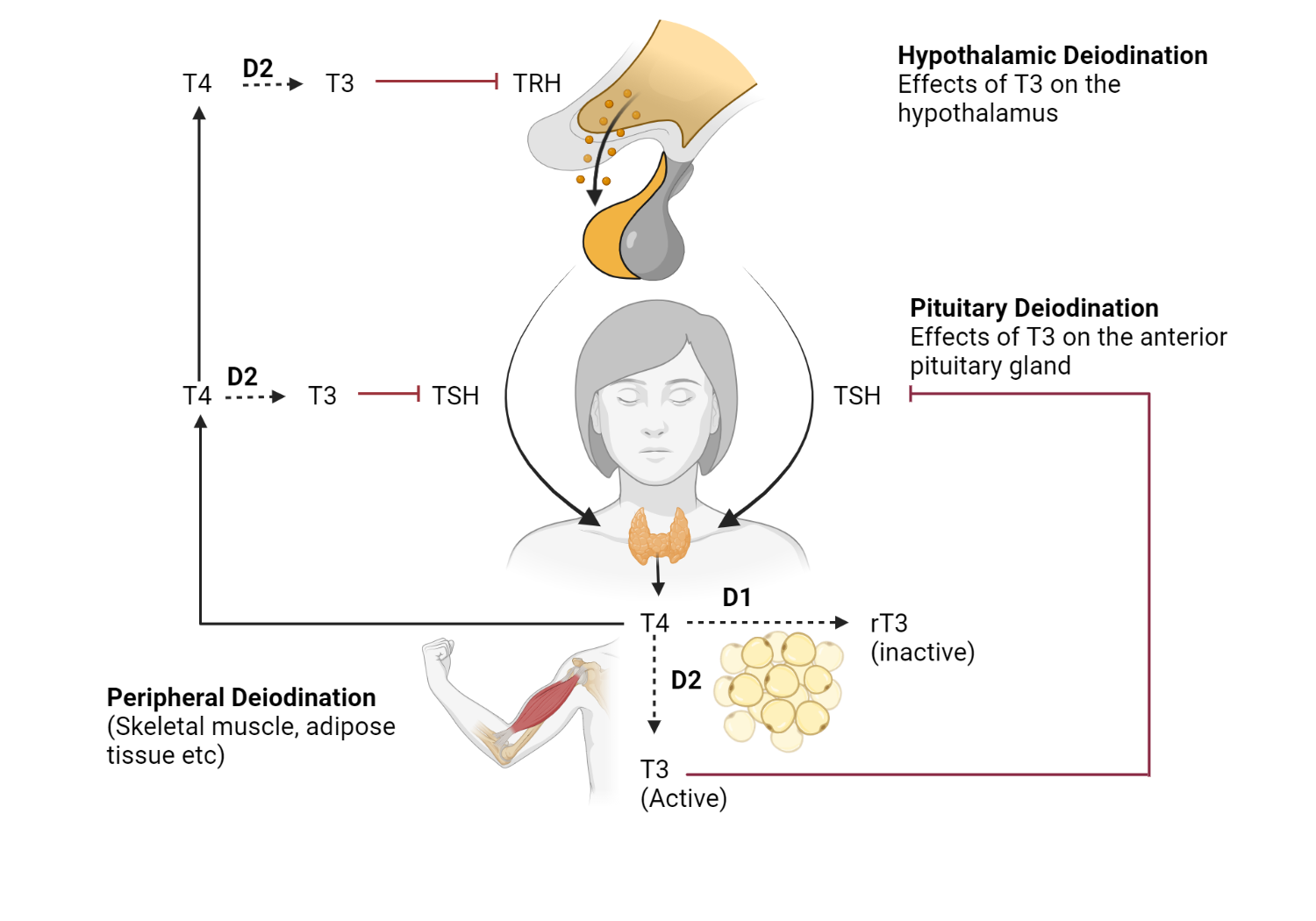
The role of deiodinases in the regulation of thyroid hormones. The role of various deiodinases (D1, D2, and D3) in regulating the hypothalamic pituitary thyroid axis. D2 is required to convert T4 to T3 not only in peripheral tissues but also in the hypothalamus and pituitary gland.
When should you consider combination T4/T3 therapy?
For patients who continue to experience subjective hypothyroid symptoms, a brief trial of a combination therapy consisting of levothyroxine and liothyronine may be considered[47,48]. However, it is crucial to rule out other potential causes of fatigue, a common complaint of hypothyroid patients, prior to initiating a trial of dual LT3 and LT4. When implementing the combination of levothyroxine (T4) and liothyronine (T3), a reasonable conversion factor is 25-50 mcg of levothyroxine to 5-10 mcg of liothyronine. For instance, the daily dose of levothyroxine can be decreased by 25 mcg when initiating liothyronine at a dose of 5 mcg, which should be administered in the morning[49].
It is important to note that for patients undergoing dual therapy, the TSH nadir, or the lowest point in the TSH levels, occurs approximately 10 hours after the administration of T3[50]. Therefore, it is recommended to monitor TSH levels early in the morning before administering the liothyronine dose. This approach will provide a more accurate assessment of the patient's thyroid hormone status and allow for any necessary adjustments in the treatment plan.
References
Kindly Let Us Know If This Was helpful? Thank You!


