This is a review of thyroid hormone physiology, including synthesis, release, the volume of distribution (role of binding proteins), and essential clinical pearls.
Thyroid Hormone Synthesis
- Iodide(I–) is transported from the perifollicular capillary (PC) into the thyroid follicular cell (FC) by the basal sodium-iodide symporter (NIS), under a sodium electrochemical gradient facilitated by the sodium-potassium pump (step 1a).
- Thyroglobulin (Tg) is synthesized from tyrosine residues, a process that occurs in the rough endoplasmic reticulum (step 1b). Tg undergoes further posttranslational modification in the Golgi apparatus of the follicular cell. It is then secreted into the follicular lumen (site of colloid storage) through a process of exocytosis.
- Iodide is transported to the apical membrane of the thyroid follicular cell, where thyroid peroxidase (TPO) enzyme catalyzes the oxidation of iodide to iodine. This oxidation step requires hydrogen peroxide(H2O2)(step 2).
- Tyrosyl residues on the thyroglobulin molecule undergo iodination by the previously oxidized iodine molecule. This process of “organification” is facilitated by TPO and results in the formation of monoiodothyronine (MIT) and diiodiothyronine (DIT) residues (step 3).
- Triiodothyronine (T3) and tetraiodothyronine (T4) are then formed in a final coupling reaction which involves the formation of ester linkages between “donor” and “acceptor” iodothyronine (MIT or DIT) residues (step 4).
- The thyroid follicular cell ingests colloid (C) by pinocytosis through the apical membrane. Proteolysis of the colloidal substrate in the thyroid follicular cell yields thyroglobulin, MIT, DIT, T3, and T4 (step 5). Deiodinases present in the follicular cell convert some T4 into active thyroid hormone (T3). Thyroid hormones (T3 and T4) are then actively transported across the basolateral plasma membrane of the thyroid epithelial cell into capillary.
Plasma protein binding and thyroid hormone levels
- Thyroid hormone are required for the differentiation, growth and metabolism of every cell in the human body. Both thyroid hormones i.e., 3,3′5-triiodo-L-thyronine (T3) and 3,3′,5,5′-tetraiodo-L-thyronine (thyroxine; T4) circulate in the blood bound to mainly plasma proteins, due to their poor water solubility.
- Indeed, only 0.4% of T4 and 0.04% of T3 (approximately a tenth of T4) exist in an unbound (or free) form in circulation[1]. In essence, these plasma binding proteins are required to maintain the large extrathyroidal pool of thyroid hormone (a storehouse for thyroid hormone).
T4 is the major thyroid hormone fraction produced by the thyroid gland. T4 is actually a pro-hormone and needs to be converted into active thyroid hormone i.e., T3. Approximately 80% of T3 is produced by deiodination in local tissues[2].
Thyroid hormone is bound to various plasma proteins, including thyroxine-binding globulin (TBG) and transthyretin (previously known as thyroxine-binding prealbumin, TBPA) and albumin. Therefore, the concentration of these plasma proteins can affect both the total and free thyroid hormone levels.
| Characteristic | TBG | TBPA | Albumin |
| Molecular weight, kDa | 54 | 55 | 66.2 |
| Binding sites | 1 | 2 | Various sites |
| % bound to T4 | 75% | 20% | 5% |
| % bound to T3 | 75% | <5% | 20% |
| Half-life (days) | 5 | 2 | 14 |
Adapted from [3]
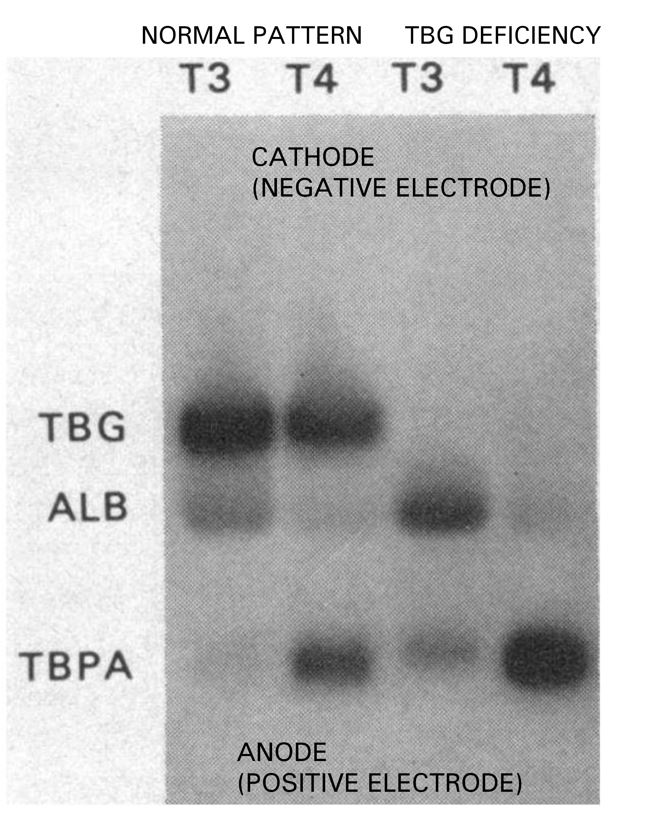
Figure 1.0. Electrophoresis pattern of thyroid hormone binding proteins in normal physiology and congenital TBG deficiency. Radiolabeled T4 and T3 added to serum subjected to electrophoresis shows that the distribution patterns for T4 and T3 differ. Although T4 binds to TBG, TBPA, and albumin, T3, on the other hand, binds to mainly TBG and albumin. Adapted from Davis et al [4]
Questions you might be asked on clinical rounds
1.) What are the consequences of changes in binding protein concentrations
Alterations in thyroid hormone-binding proteins are not associated with pathologic states (such as a change in metabolism or even overt thyroid disease). A change in the rate of synthesis and degradation of these proteins does not impact the levels of free thyroid hormone in concentration[3].
2.) What are the causes of elevated serum Total T4 in the setting of normal TSH?
This is referred to as “euthyroid hyperthyroxinemia”
Conditions that raise binding proteins
| Binding Protein | Causes |
| Increase in thyroxine-binding globulin (TBG) | Estrogen ** (hormone replacement or pregnancy) Congenital Hepatitis Tamoxifen |
| Increase in transthyretin | Congenital Paraneoplastic phenomenon (pancreatic tumors) |
| Increase in albumin | Familial dysalbuminemic hyperthyroxinemia (an inherited condition) |
** Increases the sialic acid content of the TBG molecule and thus decreasing its metabolic clearance.
Drugs that reduce the conversion of T4 to T3
An increase in T4 fraction due to its reduced conversion to active T3.
Beta-blockers, glucocorticoids, amiodarone, PTU, selenium deficiency, and iodinated contrast.
Other causes
- Patients with hypothyroidism on levothyroxine replacement therapy.
- Resistance to thyroid hormone (Refetoff syndrome)
- Heterophile anti-T4 antibody (causes antibody interference)
3.) What are the causes of low serum Total T4 in the setting of normal TSH?
This is referred to as “euthyroid hypothyroxinemia”
Conditions that reduce binding proteins.
| Binding protein | Causes |
Decrease in thyroxine-binding globulin (TBG) | Androgens Congenital Danazol Protein losses (nephrosis, chronic liver disease, enteropathy) |
| Decrease in albumin | Nephrosis Cirrhosis |
References
- Caron P (2004) Pituitary Adenomas, TSH-Secreting. In: Martini L (ed) Encyclopedia of Endocrine Diseases. Elsevier, New York, pp 624–628
- Rabah SA, Gowan IL, Pagnin M, Osman N, Richardson SJ (2019) Thyroid Hormone Distributor Proteins During Development in Vertebrates. Frontiers in Endocrinology 10:506
- Feldt-Rasmussen U, Rasmussen ÅK (2006) Thyroid Hormone Transport and Actions. In: Krassas GE, Rivkees SA, Kiess W (eds) Pediatric and Adolescent Medicine. KARGER, Basel, pp 80–103
- Davis PJ, Handwerger BS, Gregerman RI (1972) Thyroid Hormone Binding by Human Serum Prealbumin (TBPA). Electrophoretic studies of triiodothyronine-TBPA interaction. J Clin Invest 51:515–521
Kindly Let Us Know If This Was helpful? Thank You!


