Hungry Bone Syndrome (HBS) is a relatively rare but significant clinical complication that can occur after parathyroidectomy or other treatments for hyperparathyroidism. This article provides a comprehensive overview of the pathophysiology underlying HBS, focusing on the molecular mechanisms and physiological alterations that contribute to its development. Additionally, we discuss the clinical implications of HBS and its management in the context of parathyroid disorders.
A rapid and severe decline in serum calcium levels following surgical removal of hyperfunctioning parathyroid glands or other interventions for hyperparathyroidism characterizes hungry Bone Syndrome (HBS). Furthermore, HBS is primarily observed in patients with severe primary or secondary hyperparathyroidism and is associated with a high bone turnover rate and extensive bone remodeling.
This syndrome can lead to severe hypocalcemia, resulting in symptoms such as tetany, seizures, and cardiovascular complications. Understanding the pathophysiology of HBS is essential for its early recognition and effective management.
Pathophysiology of Hungry Bone Syndrome
The pathophysiology of HBS is complex and multifactorial, involving alterations in bone metabolism, calcium homeostasis, and the activity of various hormones and signaling pathways. Key aspects of the pathophysiology include:
- Pre-existing bone disease: Patients with HBS often have a history of long-standing hyperparathyroidism, which results in high bone turnover and extensive bone remodeling. This high bone turnover creates a state of increased bone resorption, with the release of calcium and phosphate from the bone matrix into the circulation. This process is primarily driven by parathyroid hormone (PTH) and receptor activator of nuclear factor kappa-B ligand (RANKL) signaling.
- Parathyroidectomy and PTH withdrawal: The surgical removal of hyperfunctioning parathyroid glands, or parathyroidectomy, leads to a rapid decline in PTH levels. The sudden withdrawal of PTH reduces osteoclastic activity and diminishes bone resorption, effectively slowing the release of calcium and phosphate from bone.
- Enhanced bone mineralization: With the decline in PTH levels, osteoblastic activity becomes more pronounced, leading to enhanced bone formation and mineralization. This process results in a substantial influx of calcium and phosphate ions into the bone matrix, effectively “trapping” these ions and reducing their availability in the circulation.
- Increased calcium demand: The rapid mineralization of bone creates a state of high calcium demand, leading to the “hungry bone” phenomenon. This increased demand for calcium outpaces the supply, resulting in a rapid decline in serum calcium levels and the development of hypocalcemia.
- Altered vitamin D metabolism: Patients with hyperparathyroidism often have altered vitamin D metabolism, leading to reduced levels of the active form of vitamin D, calcitriol (1,25-dihydroxyvitamin D3). Calcitriol is essential for intestinal calcium absorption, and its deficiency can contribute to the development of hypocalcemia following parathyroidectomy.
- Inadequate compensatory mechanisms: In HBS, the compensatory mechanisms that usually help maintain calcium homeostasis, such as increased renal calcium reabsorption and enhanced intestinal calcium absorption, are insufficient to counteract the rapid decline in serum calcium levels.
Biochemical features of HBS
- Hypocalcemia
- Hypophosphatemia
- Hypomagnesemia
- raised alkaline phosphatase
- hypocalciuria
Etiology
- Parathyroidectomy for hyperparathyroidism (primary/secondary/tertiary)
- rickets/osteomalacia who are replaced with vitamin D alone
without concomitant calcium - Untreated severe hyperthyroidism following thyroidectomy
- Acute correction of metabolic acidosis in patients with renal tubular acidosis
- Institution of antiresorptive therapy in patients with osteoblast metastasis (e.g., carcinoma prostate).
- Cushing’s syndrome may also develop HBS after curative surgery
What are the likely predictors of HBS?
- Age (>60 years)
- Menopausal syndrome (hypoestrogenemia)
- Hypovitaminosis D
- Primary hyperparathyroidism complicated by osteitis fibrosa cystica( elevated serum alkaline phosphatase and brown tumors)
- Moderate to severe preoperative hypercalcemia in the setting of primary hyperparathyroidism
- Giant parathyroid tumors (>5 cm)
Preventive Strategies
Management strategies for HBS include:
- Pre-operative evaluation: A thorough pre-operative assessment, including the evaluation of bone mineral density, serum calcium, phosphate, PTH, and vitamin D levels, can help identify patients at risk of developing HBS.
- Prophylactic measures: In patients with a high risk of HBS, prophylactic measures, such as optimizing vitamin D and calcium levels before surgery, can help reduce the incidence and severity of postoperative hypocalcemia.
- Intraoperative PTH monitoring: Intraoperative PTH monitoring can provide valuable information about the adequacy of parathyroid gland removal and may help predict the risk of postoperative hypocalcemia, allowing for early intervention and management.
- Postoperative monitoring: Close monitoring of serum calcium, phosphate, magnesium, and PTH levels is crucial in the early postoperative period to identify and manage HBS. Electrocardiographic monitoring may be necessary in patients with severe hypocalcemia to detect and manage potential cardiac complications.
- Calcium and vitamin D supplementation: Oral or intravenous calcium supplementation, along with active vitamin D analogs (e.g., calcitriol), can be used to manage hypocalcemia in HBS patients. The dosage and duration of treatment should be tailored to the patient’s needs and adjusted based on serum calcium levels.
- Magnesium replacement: Hypomagnesemia can impair PTH release and exacerbate hypocalcemia in HBS patients. Therefore, monitoring and correcting magnesium levels are essential components of HBS management.
- Bisphosphonates: In select cases, bisphosphonates may be used as an adjunct therapy to reduce bone turnover and minimize the risk of HBS. However, the use of bisphosphonates in HBS patients should be carefully considered, as their effects on bone metabolism can be long-lasting and may interfere with bone remodeling in the long term.
Pathophysiology Pearls
Will Hungry Bone Syndrome develop in patients with primary hyperparathyroidism who develop post-operative hypoparathyroidism?
An elevated serum PTH is required for bone remodeling in the immediate postoperative period. As a consequence, patients with hypoparathyroidism in the postoperative period are unlikely to develop hungry bone syndrome
What is the role of PTH in osteoblast-osteoclast interaction?
In normal physiology, PTH binds to osteoblasts and activates them through complex downstream processes. An activated osteoblast’s surface-bound receptor activator of nuclear factor κ-B ligand (RANK-L) binds to receptor activator of nuclear factor κ-B (RANK) present on the surface of the osteoclast. This promotes osteoclast activation and subsequently leads to increased bone resorption. PTH also suppresses the synthesis of osteoprotegerin (OPG) – a soluble decoy receptor for RANK-L, which results in a higher number of RANK-Ls being available to bind osteoclast surface-bound RANK
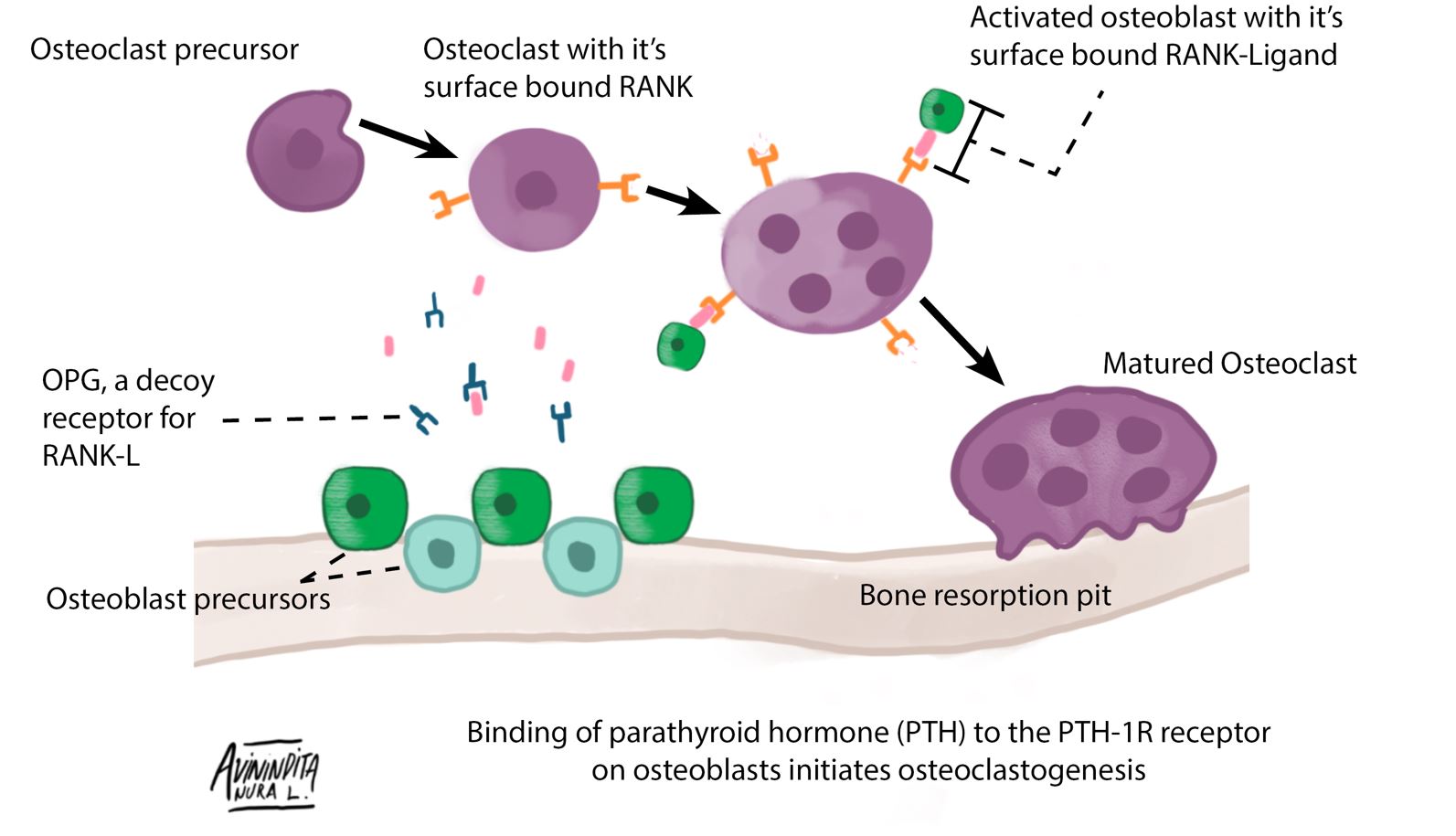
PTH binds to the PTH-1R (Parathyroid hormone 1 receptor) on osteoblasts; this is the first step in the eventual activation of osteoclasts. Osteoblast surface-bound RANK-L binds to RANK on osteoclasts leading to differentiation of an osteoclast precursor into a mature osteoclast. Mature osteoclasts present in bone resorption pits are responsible for the liberation of calcium sequestered in hydroxyapatite crystals. Osteoprotegerin is a soluble decoy receptor for RANK-L, which provides negative feedback inhibition of osteoclast activation. (Redrawn and modified from Ikeda et al. (2016) The role of osteoclast differentiation and function in skeletal homeostasis. J Biochem 159:1–8
Conclusion
Hungry Bone Syndrome is a complex and multifactorial condition resulting from the interplay of various factors, including alterations in bone metabolism, calcium homeostasis, and hormonal signaling. A comprehensive understanding of its pathophysiology is essential for early recognition, effective management, and the prevention of serious complications. By adopting a proactive approach to the evaluation and management of at-risk patients, healthcare professionals can mitigate the impact of HBS and improve patient outcomes following parathyroidectomy or other interventions for hyperparathyroidism.
References
- Pesce CE, Shiue Z, Tsai HL, Umbricht CB, Tufano RP, Dackiw AP, Kowalski J, Zeiger MA. Postoperative hypocalcemia after thyroidectomy for Graves’ disease. Thyroid. 2010 Nov 1;20(11):1279-83.
- Alfaro Riveros H, Almodóvar LO, Farriols Danés C, Domingo JP. Hungry bone syndrome: persistent hypocalcemia related to osteoblastic bone metastases of prostate cancer. Journal of Palliative Medicine. 2013 Dec 1;16(12):1496-7.
- Jain N, Reilly RF. Hungry bone syndrome. Current opinion in nephrology and hypertension. 2017 Jul 1;26(4):250-5.
Kindly Let Us Know If This Was helpful? Thank You!


