In adult humans, the body contains approximately 1000 g of calcium (Ca), with 99% present in the mineral phase of bone as crystals of hydroxyapatite [Ca10 (PO4) 6 (OH) 2]. These crystals significantly contribute to the mechanical weight-bearing properties of bone and act as a readily available Ca source for various Ca-dependent biological systems and maintain blood-ionized Ca within normal ranges. The remaining 1% of total body Ca is found in blood, extracellular fluid (ECF), and soft tissues.
Calcium Levels In Cells
The cytoplasmic Ca concentration is approximately 10^−6 M, while the ECF Ca concentration is 10^−3 M, creating a 1000-fold gradient across the plasma membrane that favors Ca entry into the cell. Furthermore, an electrical charge of about 50 mV exists across the plasma membrane, with the cell interior being negative. The chemical and electrical gradients across the plasma membrane support Ca entry, which the cell must counteract to preserve cell viability. Multiple mechanisms, including Ca pumps and channels, Na-Ca exchangers, and intracellular Ca binding by proteins, prevent Ca-induced cell death. These Ca-binding sites within the cytoplasm, endoplasmic reticulum (ER), and mitochondria buffer intracellular Ca, which can be mobilized to maintain cytoplasmic Ca levels and create pulsatile Ca peaks to mediate membrane receptor signaling and regulation of various biological systems.
Calcium Levels In Blood
The free or ionized fraction (45%) of total blood Ca is biologically functional and clinically measurable, while 45% of the total is bound to albumin in a pH-dependent manner and the remaining 10% forms complexes with anions such as phosphate (PO4) and citrate. Although only ionized Ca is available for cellular processes, most clinical laboratories report total serum Ca concentrations. Normal serum concentrations of total Ca generally range between 8.5 and 10.5 mg/dL (2.12 to 2.62 mM), and levels above this range are considered hypercalcemic. The reference range for ionized Ca is 4.65 to 5.25 mg/dL (1.16 to 1.31 mM). Fluctuations in protein concentrations, particularly albumin concentrations, may cause variations in total Ca levels, while ionized Ca may remain relatively stable. Dehydration or hemoconcentration during venipuncture can increase serum albumin and falsely increase total serum Ca. Adjustments for elevated or reduced total Ca can be made, taking into account albumin levels and the impact of changes in blood pH on the equilibrium constant of the albumin-Ca^2+ complex.
Calcium Homeostasis And Balance
The ECF Ca concentration is tightly maintained within a narrow range due to the importance of Ca ions in numerous cellular functions, such as cell division, cell adhesion, plasma membrane integrity, protein secretion, muscle contraction, neuronal excitability, glycogen metabolism, and coagulation. Skeletal, intestinal, and kidney play a major role in ensuring Ca homeostasis. In a typical individual, about 200 mg of ingested Ca (1000 mg/day) will be absorbed, with approximately 10 g of Ca filtered daily through the kidney and most reabsorbed, resulting in about 200 mg excreted in the urine. The skeleton, the primary Ca reservoir in the body, stores approximately 1 kg of Ca.
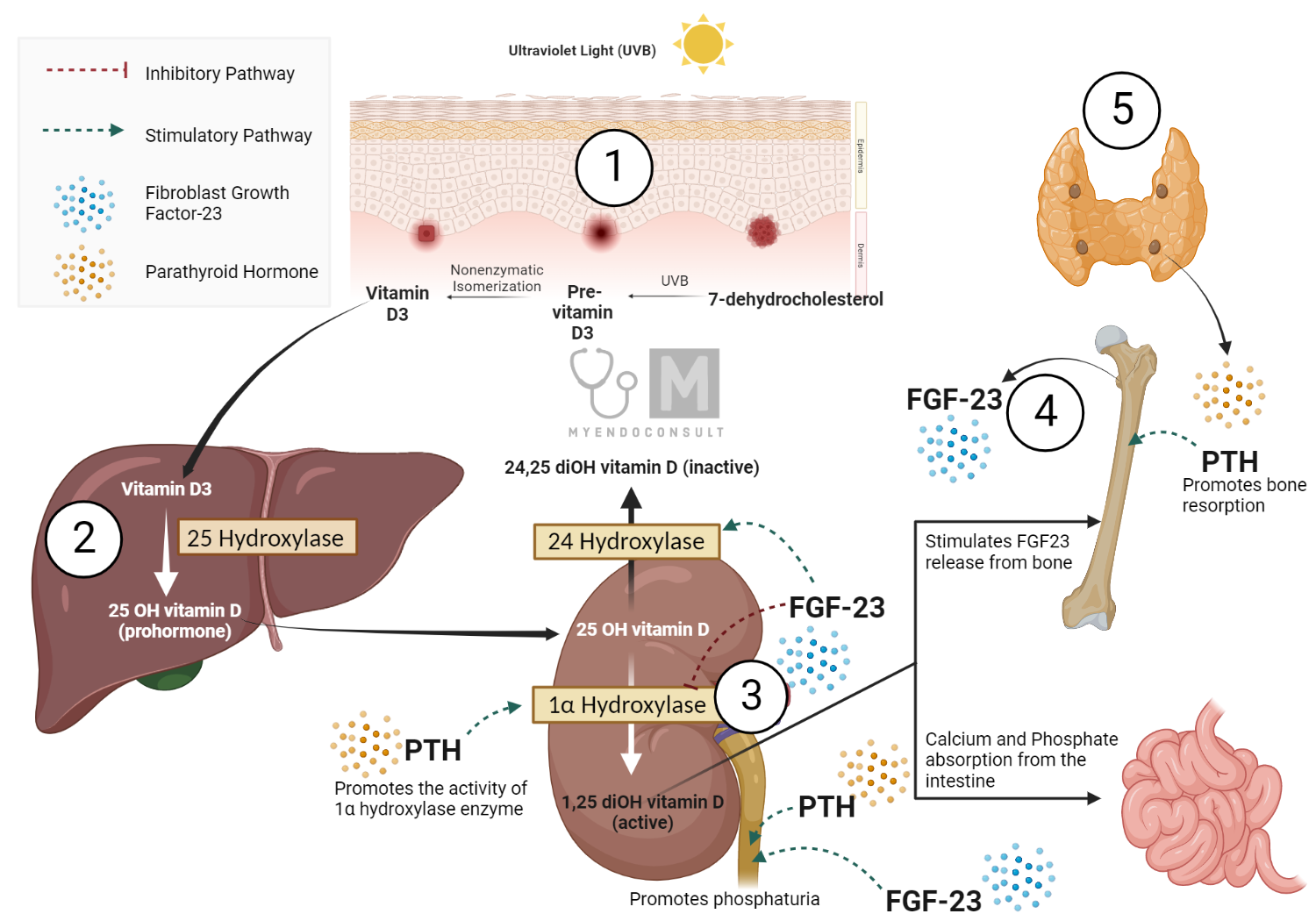
Regulation of Hormone Production in Calcium Homeostasis
Parathyroid Hormone (PTH) Production
Extracellular fluid (ECF) calcium (Ca) concentration plays a crucial role in the regulation of PTH secretion by the parathyroid glands. Calcium-sensing receptors (CaSRs), which are Gq protein-coupled receptors, detect Ca levels within parathyroid chief cells. A decrease in ECF Ca leads to an increase in PTH secretion. The relationship between ECF Ca and PTH secretion follows a steep inverse sigmoidal curve, characterized by a maximal secretory rate at low ECF Ca, a midpoint or a "set point," and a minimal secretory rate at high ECF Ca. Sustained hypocalcemia can result in proliferation of parathyroid cells and increased total secretory capacity. ECF Ca, acting via CaSR, functions as a hormone that modulates PTH release and parathyroid cell function. Furthermore, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) suppresses PTH synthesis and proliferation of parathyroid cells.
Vitamin D Production and Metabolism
Vitamin D, produced in the skin or ingested through the diet, is 25-hydroxylated in the liver, creating the 25OHD metabolite. The active form, 1,25(OH)2D, is formed in the kidney and other tissues by the mitochondrial enzyme 25OHD-1α hydroxylase (CYP27B1 or 1α(OH)ase). Renal production of 1,25(OH)2D is stimulated by hypocalcemia, hypophosphatemia, and elevated levels of PTH. The renal 1α(OH)ase is inhibited by 1,25(OH)2D through a negative feedback loop. Furthermore, 1,25(OH)2D can stimulate a renal 24-hydroxylase enzyme (CYP24A1 or 24(OH)ase) to convert 25OHD and 1,25 (OH) 2D to inactive forms. Fibroblast growth factor 23 (FGF23) inhibits renal 1α(OH)ase and stimulates renal 24(OH)ase, acting as a counter-regulatory hormone for the effects on mineral homeostasis.
FGF23 production
FGF23 production can be stimulated by local and systemic factors, including high dietary phosphorus, low dietary phosphorus, serum calcium, serum phosphorus, PTH, and 1,25(OH)2D. FGF23 and 1,25(OH)2D participate in an endocrine loop in which 1,25(OH)2D stimulates FGF23 production and FGF23 suppresses 1,25(OH)2D levels.
Intestinal Calcium Transport
Net intestinal calcium absorption can be determined using the external balance technique. The efficiency of calcium absorption varies with age and the amount ingested. 1,25(OH)2D3 increases the efficiency in absorbing dietary calcium. Intestinal epithelial calcium transport includes an active energy-dependent cell-mediated process regulated by 1,25(OH)2D and a passive, diffusional paracellular path of absorption driven by transepithelial electrochemical gradients. Active transcellular intestinal absorption involves TRPV6, calbindin-D9K, and PMCA1b.
25(OH)2D can also regulate paracellular calcium channels and reroute Ca through paracellular epithelial cell junctions. Passive diffusion increases linearly with luminal Ca concentration, and during high dietary Ca intake, 1,25(OH)2D is suppressed, leading to almost all absorption being attributed to passive paracellular transport. Various factors can cause increased or decreased intestinal Ca absorption.
In summary, regulation of hormone production in calcium homeostasis is a complex interaction between PTH, 1,25(OH)2D, calcium, and FGF23, which act on target tissues to maintain calcium balance. PTH production is regulated by Ca levels, while vitamin D metabolism and FGF23 production are influenced by dietary factors, PTH, and 1,25(OH)2D levels. Intestinal calcium transport involves both active transcellular and passive paracellular pathways, with 1,25(OH)2D playing a crucial role in modulating the efficiency of these processes. Understanding the molecular details of these pathways is essential to understand the mechanisms underlying calcium homeostasis and to develop effective treatments for related disorders.
Renal Calcium Transport
The kidney is crucial in maintaining calcium balance, with calcium from PTH and ECF playing major roles in fine-tuning renal function. PTH has a limited effect on calcium fluxes in the proximal tubule, where 65% of the filtered calcium is reabsorbed. PTH can stimulate 1α(OH)ase, increasing the synthesis of 1,25(OH)2D. Reduced ECF calcium can also stimulate 1,25(OH)2D production, although the role of CaSR is unclear.
PTH can inhibit sodium and bicarbonate reabsorption in the proximal tubule by inhibiting apical type 3 Na+/H+ exchanger and basolateral Na+/K+‐ATPase, as well as inhibiting apical Na+/PO4‐ cotransport by inhibiting type II Na + ‐dependent phosphate cotransporters NaPi‐IIa and NaPi‐IIc. About 20% of the filtered calcium is reabsorbed in the thick ascending limb of the loop of Henle (CTAL) and 15% in the convoluted distal convoluted tubule (DCT). PTH enhances calcium reabsorption at both sites by binding to the PTH receptor (PTHR).
In the CTAL, increased ECF calcium can reduce the activity of the Na/K/2Cl cotransporter and decrease paracellular calcium reabsorption. This antagonizes the effect of PTH on this nephron segment and helps regulate calcium homeostasis. In the DCT, PTH influences luminal calcium transfer into the renal tubule cell via TRPV5, translocation of calcium across the cell, and active extrusion of calcium from the cell into the blood via a Na+/Ca exchanger, NCX1.
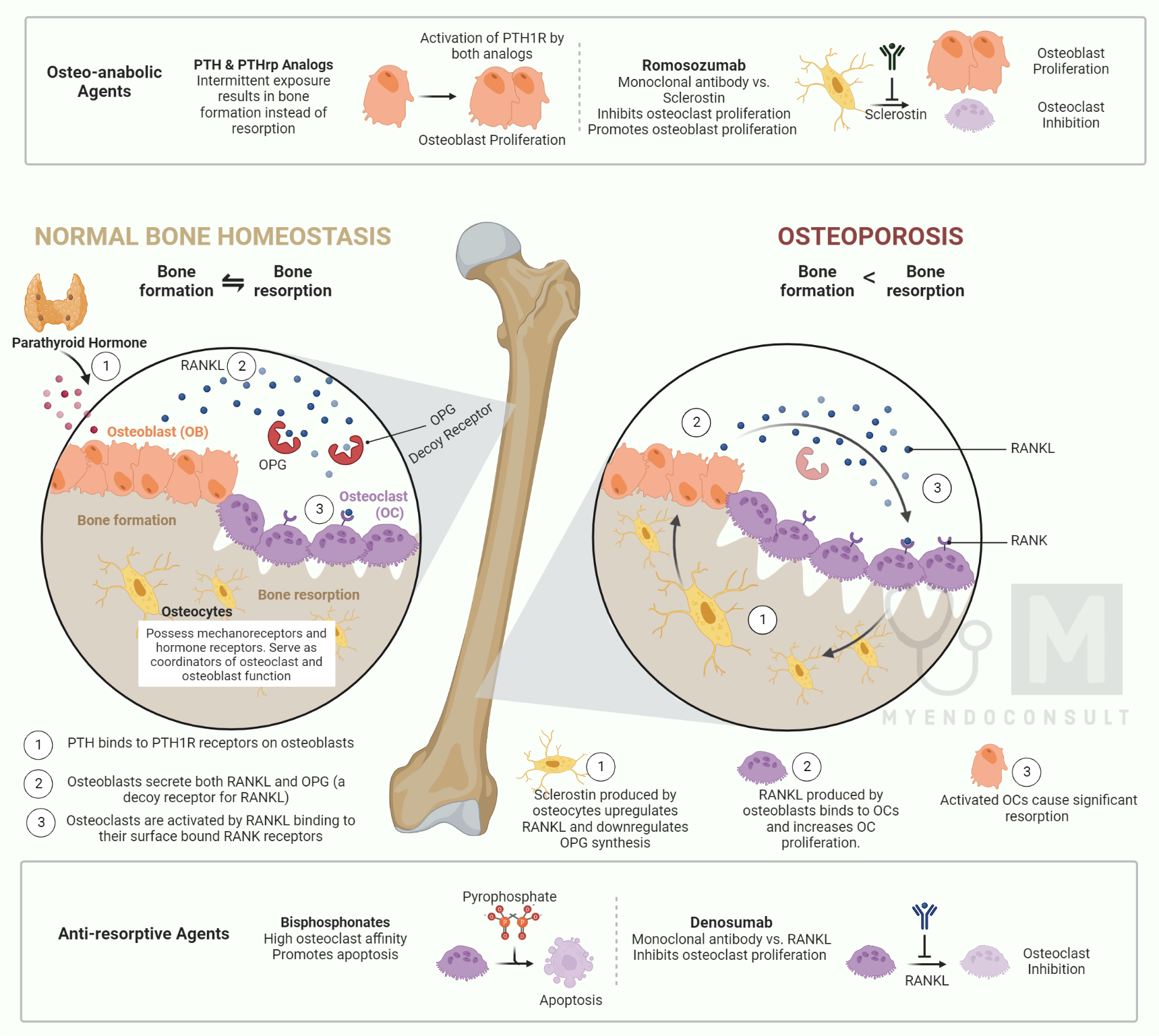
Skeletal Regulation of Calcium Homeostasis
In bone, the PTHR is present on osteoblasts but not on osteoclasts. PTH helps maintain normal calcium homeostasis by increasing the release of the cytokine RANKL, which promotes osteoclast formation and activity. PTH may also reduce osteoprotegerin, inhibiting osteoclastic activity. Vitamin D is essential for normal bone mineralization, indirectly improving intestinal calcium and phosphate absorption. A major direct function of 1,25 (OH) 2D in bone is to enhance calcium store mobilization when dietary calcium is not sufficient. Similarly to PTH, 1,25 (OH) 2D improves osteoclastic bone resorption by binding to receptors on osteoblastic cells and increasing the RANKL/OPG ratio. High levels of 1,25(OH)2D may also inhibit mineralization. Both endogenous and exogenous 1,25(OH)2D3 play been reported to have an anabolic role in vivo.
The regulation of calcium homeostasis follows a temporal sequence. When hypocalcemia occurs, there is an elevation in circulating PTH levels, which increases distal renal tubular calcium reabsorption within minutes. There may also be PTH-independent "buffering" of ECF calcium by bone through mechanisms that are not yet fully understood, potentially involving the CaSR. This rapid response helps return the calcium from the ECF to its baseline value after hypocalcemia. Short periods of hypocalcemia can be corrected solely through increased renal calcium conservation and mobilization of calcium from bone.
PTH-induced osteoclastic bone resorption likely occurs after several hours to days, and PTH-induced stimulation of renal synthesis of 1,25(OH)2D from 25OHD takes several hours. More prolonged hypocalcemia and exposure to elevated PTH may involve an increase in intestinal calcium absorption, as well as a calcium release of calcium from bone.
In general, an elevation in circulating PTH in response to hypocalcemia is sufficient to restore normocalcemia within minutes to a few hours. However, in some clinical conditions (eg significantly low calcium intake or vitamin D deficiency), greater and more prolonged increases in PTH levels are necessary to restore and maintain normocalcemia. This can be achieved through a temporal-graded series of responses of the parathyroid glands to low calcium from the ECF and/or associated deficiency of 1,25(OH)2D.
Following the initial release of stored PTH in response to hypocalcemia, which occurs in seconds and lasts for 60 to 90 minutes, there is decreased intracellular degradation of PTH within 20 to 30 minutes, increased expression of the PTH gene over hours, and eventually increased proliferation of parathyroid cells over weeks to months or more. In situations such as chronic kidney disease and severe hyperparathyroidism, there can be large increases in the circulating PTH and the parathyroid cellular mass.
Summary and Conclusion
In conclusion, calcium homeostasis is a complex and essential process that ensures the proper functioning of numerous physiological processes in the human body. The delicate balance of calcium levels is maintained through the intricate interplay between the parathyroid hormone (PTH), vitamin D, and the calcium-sensing receptor (CaSR). These factors regulate calcium metabolism in the kidneys, intestine, and bone, which are the primary organs involved in maintaining calcium balance.
The kidney plays a crucial role in regulating calcium levels by modulating calcium reabsorption and handling through the actions of PTH and vitamin D. The intestine, under the influence of 1,25(OH)2D, is responsible for dietary calcium absorption, while bone acts as a reservoir for calcium, with osteoclasts and osteoblasts regulating bone resorption and formation, respectively.
The temporal sequence of calcium homeostasis regulation involves rapid responses to hypocalcemia, such as increased renal calcium reabsorption and PTH-independent "buffering" by bone, followed by slower processes, including PTH-induced osteoclastic bone resorption and renal synthesis of 1,25(OH)2D. In certain clinical conditions, more prolonged increases in PTH levels are needed to maintain calcium balance.
Understanding the mechanisms of calcium homeostasis is vital for diagnosing and treating various disorders related to calcium imbalance, such as hypocalcemia, hypercalcemia, and osteoporosis. The knowledge of these processes also allows for the development of therapeutic interventions that target specific molecular pathways, improving patient outcomes in calcium-related disorders.
In summary, calcium homeostasis is a highly regulated and interconnected process that involves the concerted action of hormones, receptors, and organs to maintain the appropriate balance of calcium in the body. Continued research and understanding of these intricate mechanisms will contribute to the advancement of medical knowledge and the development of new treatments for calcium-related disorders.
Kindly Let Us Know If This Was helpful? Thank You!


