Corticosteroids are a class of steroid hormones produced by the adrenal cortex that play diverse physiological roles in the body. They include glucocorticoids, mineralocorticoids, and androgens.
Glucocorticoids are a family of steroid hormones that are synthesized from the adrenal cortex that play a pivotal role in the treatment of inflammation, cancer, and autoimmune diseases. Furthermore, glucocorticoids play an essential role in the metabolism of fat, protein, and glucose in the body.
Synthesis of glucocorticoids occurs primarily in the cortex of the adrenal gland alongside dehydro-epi-androsterone (DHEA) and aldosterone (a mineralocorticoid). DHEA is the precursor of estrogen and testosterone. DHEA, glucocorticoids, and aldosterone are synthesized in the mitochondria by different steroidogenic enzymes. But they share one thing in common – they are synthesized from cholesterol. Interestingly, various studies have reported extra-adrenal glucocorticoid production in the vasculature, thymus, epithelial barriers, and the brain.
The medical significance of glucocorticoids arises from their anti-allergic, immune-suppressive, and anti-inflammatory role in the body. It is worth mentioning that the immune-suppressive role of glucocorticoids is pivotal in medical treatment. It is also worth noting that glucocorticoids are not the anabolic steroids used for muscle buildup, but are rather catabolic steroids that trigger the breakdown of peripheral muscle.
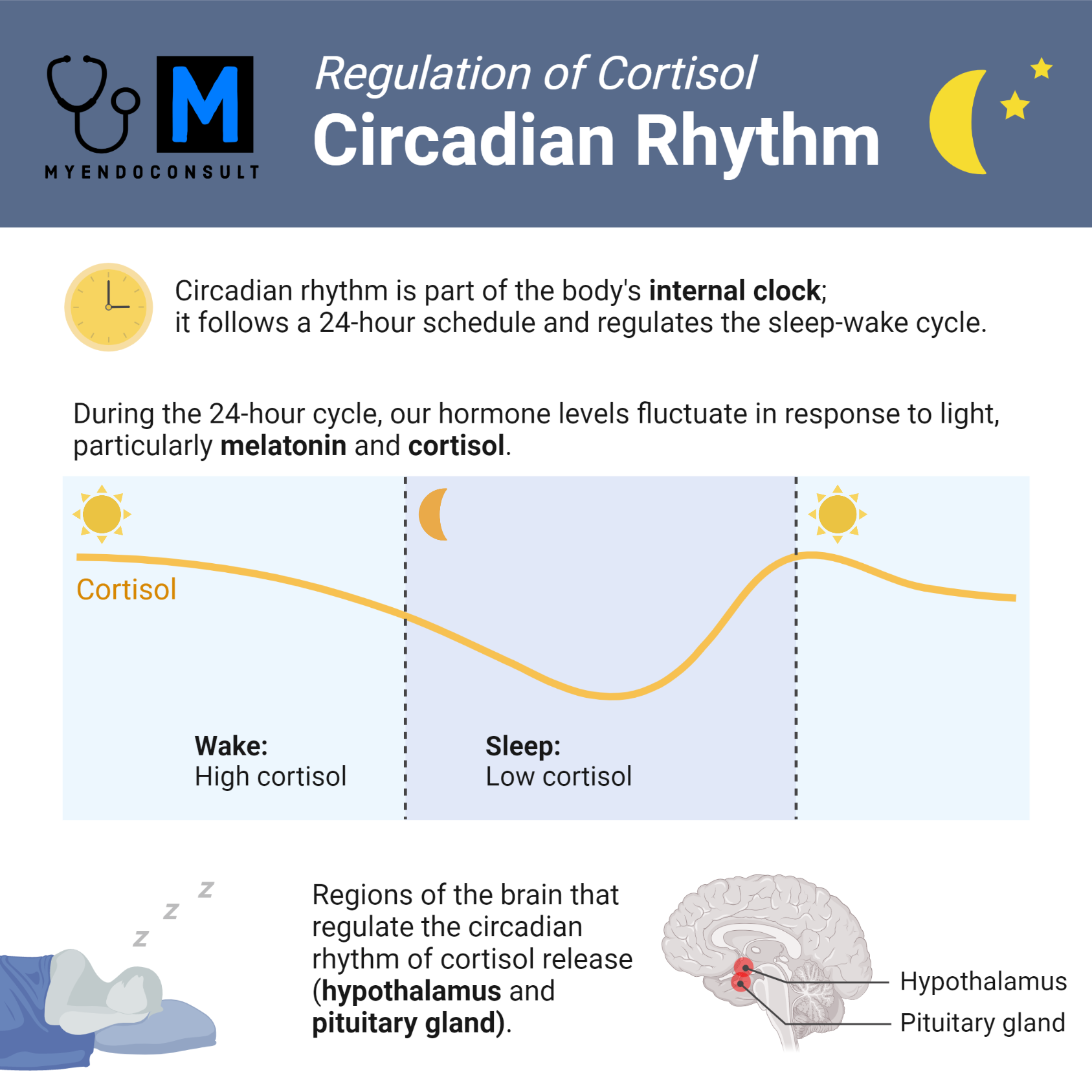
Cortisol is the essential glucocorticoid in the human body. Its secretion occurs in a diurnal circadian pattern, with the lowest levels released between 12am (midnight) and 4am and the peak levels released around 8am. This article will highlight the different types of glucocorticoids and their physiological role in the human body.
Synthesis & release of glucocorticoids
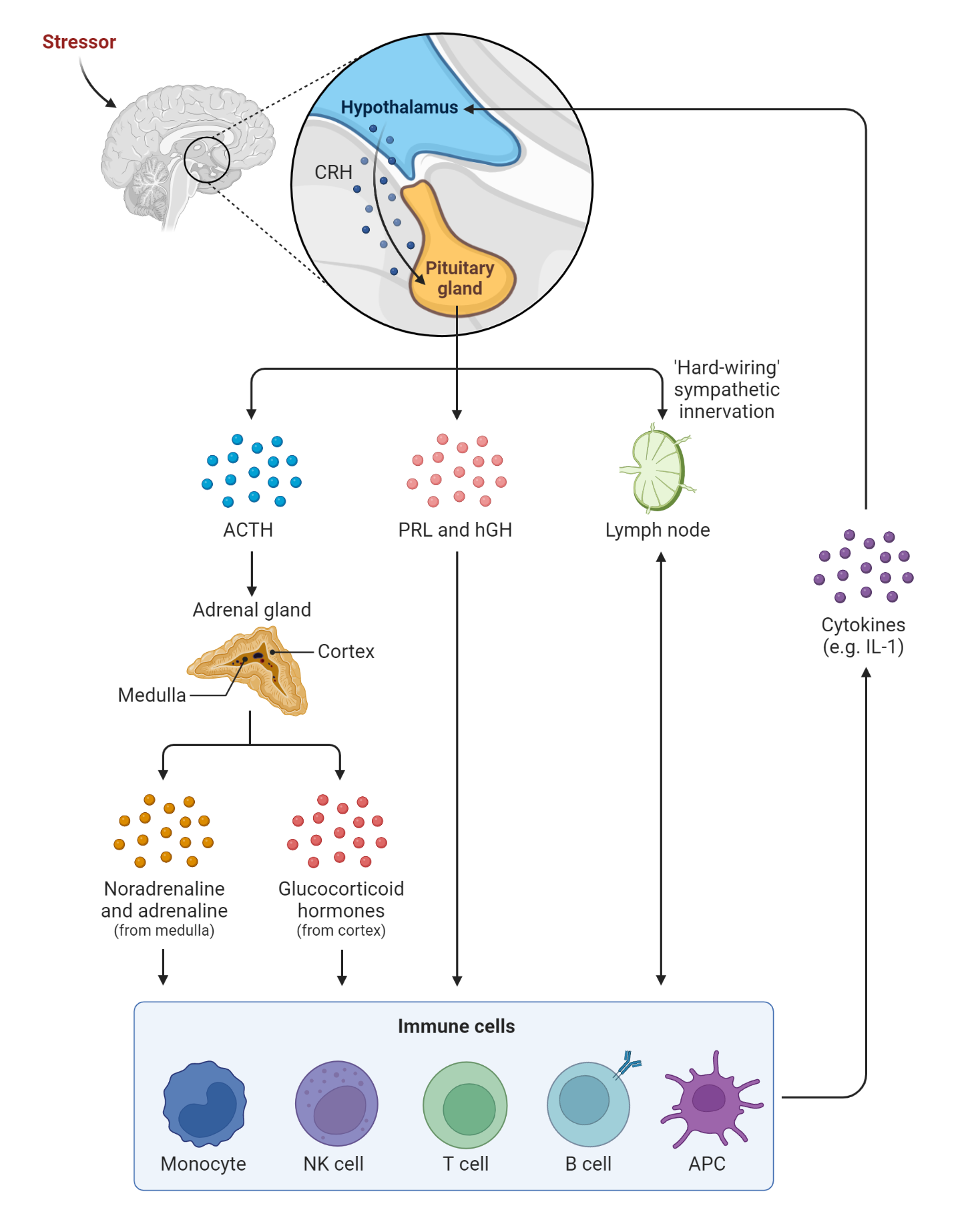
Glucocorticoids (known as corticosterone in rodents and cortisol in humans) are synthesized in the adrenal glands and released in a circadian manner. The release of glucocorticoids occurs in response to stress and physiological cues.1
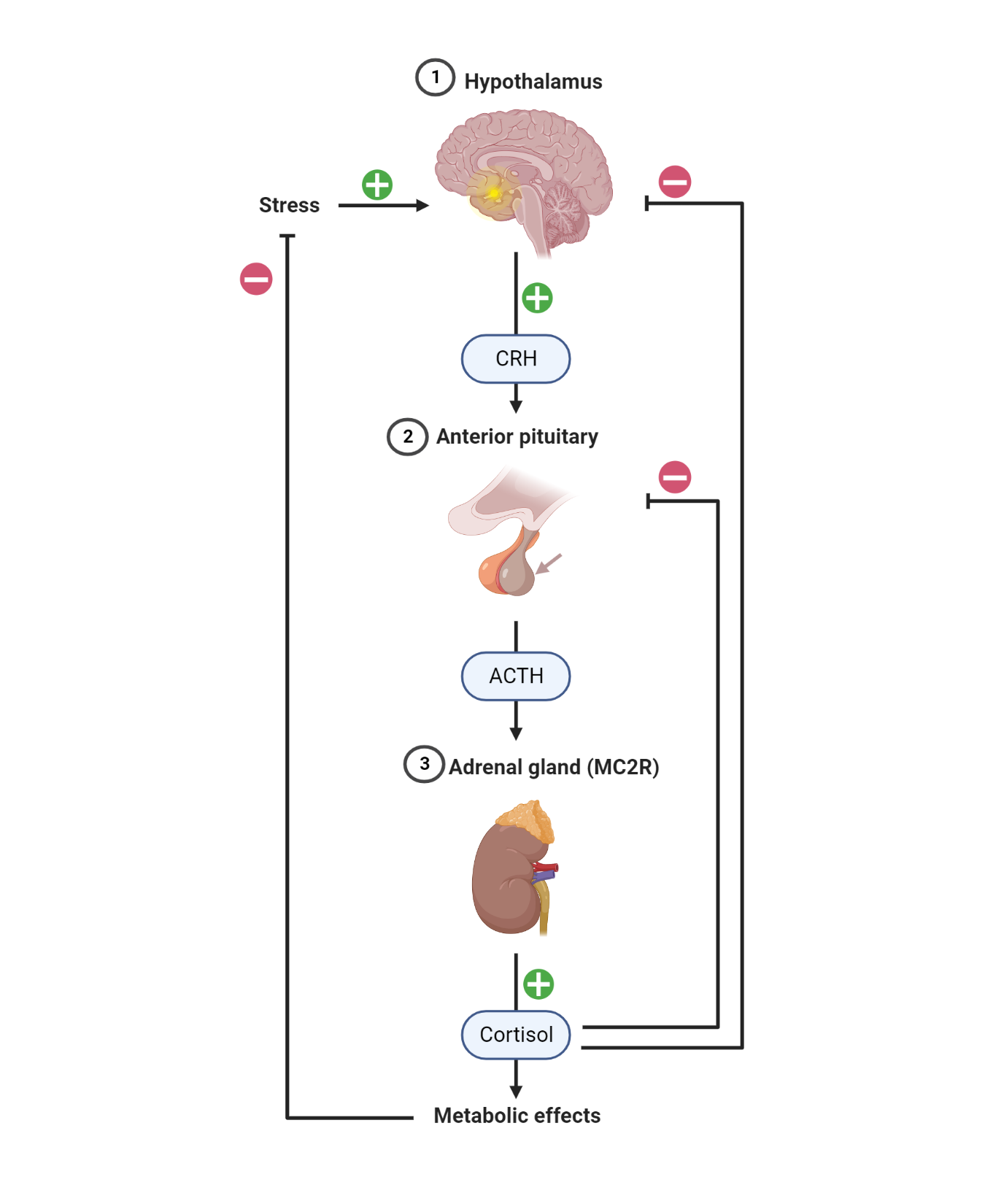
Cortisol release is controlled by the hypothalamic-pituitary-adrenal (HPA) axis. Corticotrophin-releasing hormone (CRH) is secreted from the hypothalamus. CRH stimulates then stimulates the production of adrenocorticotrophic hormone (ACTH). ACTH subsequently binds to the melanocortin two receptor – MC2R, a G protein-coupled receptor. The binding of ACTH to MC2R activates adenylyl cyclase, production of cAMP, and protein kinase A (PKA) activation. PKA modifies the activity of certain transcription factors via phosphorylation. As a result, the modified transcription factors can bind to specific DNA sequences in the nucleus, such as the cAMP response element (CRE), and stimulate the transcription of genes involved in glucocorticoid synthesis, such as the steroidogenic acute regulatory protein (StAR) and cytochrome P450 enzymes (CYP11A1 and CYP17A1). This leads to the increased production and release of glucocorticoids, such as cortisol, from the adrenal gland into the circulation
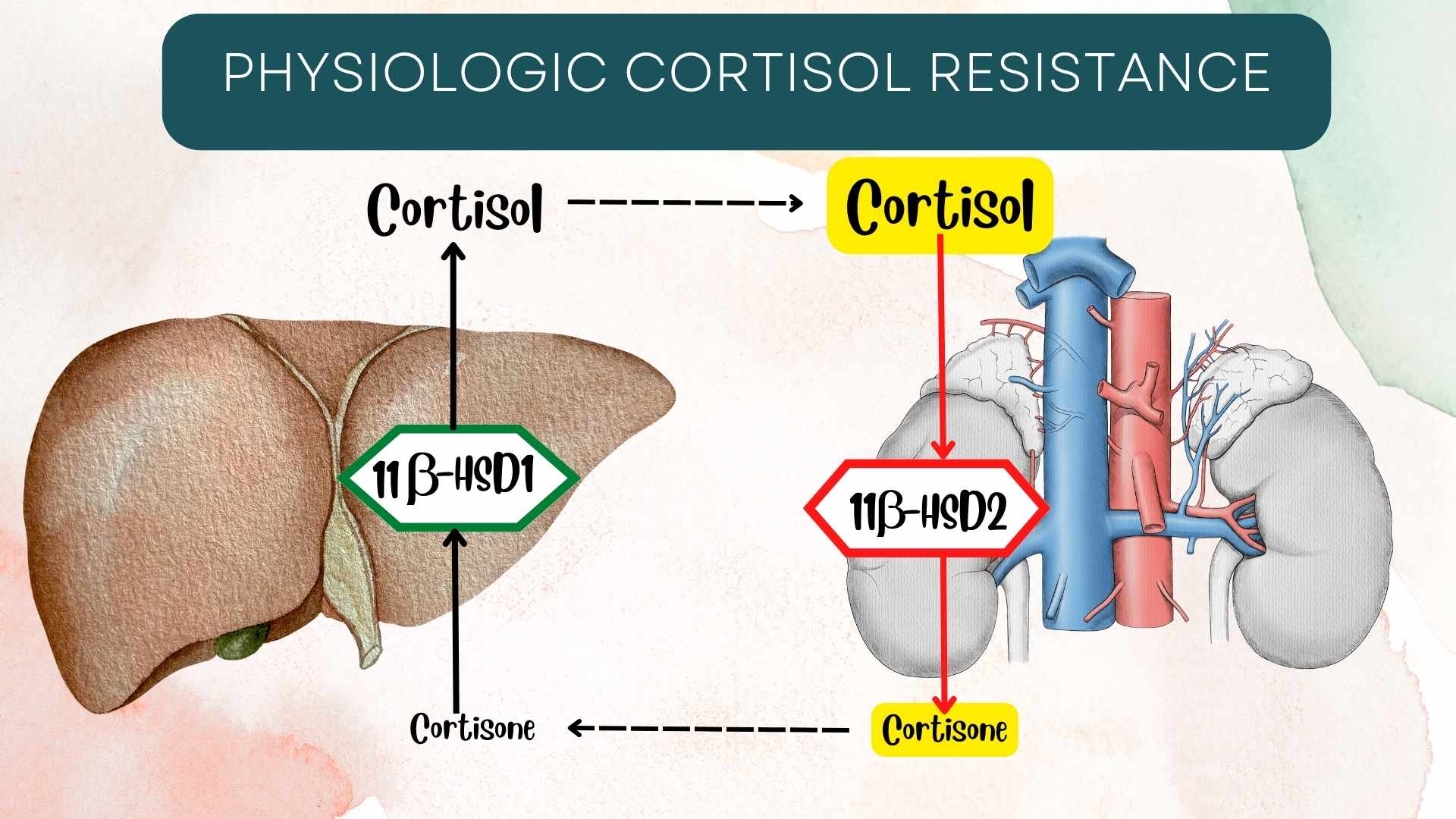
The production of glucocorticoid in zona fasciculata involves 11β-hydroxylase, 17a-hydroxylase, 21-hydroxylase, and desmolase. The end product is cortisone. Cortisone is the inactive form of cortisol. Cortisone is converted in the liver to cortisol. The conversion is facilitated by type 1 hydroxysteroid dehydrogenase. The activated cortisol is bound to cortisol binding protein, a globulin protein (produced by the liver) and released in the blood.2 It is important to note that cortisol has the potential to bind to and activate both glucocorticoid and aldosterone receptors under physiological conditions. 11-β hydroxysteroid (type II) dehydrogenase prevents the activation of mineralocorticoid receptors by converting cortisol to cortisone (the inert form of cortisol). However, 11β-hydroxysteroid dehydrogenase becomes saturated when cortisol levels are very high, thus activating mineralocorticoid receptors. This is usually due to treatment with supra-physiologic doses of exogenous glucocorticoids.3
After binding to a glucocorticoid receptor, the cortisol-GR complex is translocated inside the nucleus. The compound alters gene expression and induces various effects on the body cells like white blood cells, fibroblasts, etc.4 Cortisol has a half-life of 66 minutes under physiological conditions.
https://youtu.be/x38_iiSoc98
Regulation of endogenous glucocorticoid production
The production of glucocorticoids is also subject to negative feedback regulation. Elevated levels of glucocorticoids in the blood inhibit the secretion of CRH and ACTH, reducing the production of glucocorticoids. This negative feedback system helps to maintain the appropriate level of glucocorticoids in the body.
Other hormones also play a role in the regulation of glucocorticoid production. For example, the hormone vasopressin, which is secreted by the hypothalamus, can enhance the release of CRH (a CRH secretagogue), thereby increasing the production of glucocorticoids. Similarly, the hormone growth hormone can enhance the responsiveness of the adrenal gland to ACTH, leading to increased production of glucocorticoids.
Disruptions in the regulation of glucocorticoid production can lead to a range of disorders. For example, adrenal insufficiency occurs when the adrenal gland fails to produce adequate levels of glucocorticoids, resulting in symptoms such as fatigue, weight loss, and hypotension. On the other hand, Cushing’s syndrome occurs when the body produces too much glucocorticoid, leading to symptoms such as weight gain, hypertension, and osteoporosis.
Physiological function of Glucocorticoids
Glucocorticoids are released into the general circulation in a pulsatile pattern, peaking around 8am. Glucocorticoids play a vital role in bodily functions. Stress induces cortisol secretion. Its role in stress is the reason it is identified as the body’s stress hormone. 5
Glucocorticoid role in metabolism
Cortisol’s action on glucose metabolism is the underlying cause of hyperglycemia. This metabolic effect not only regulates glucose homeostasis, but also provides energy to the body’s vital organs during times of stress (for instance, during exercise or illness). Hyperglycemia is caused by increasing the synthesis of enzymes that are involved in gluconeogenesis and glycogenolysis.6
Cortisol induces, activates or upregulates the enzymes that are involved in glycogenolysis and gluconeogenesis. Cortisol works as an insulin antagonist and decreases the uptake of glucose by the cells to boost the availability of glucose for the red blood cells, brain, and the skeletal muscles. Cortisol induces Phosphoenolpyruvate carboxykinase (PEPCK) enzyme expression which ultimately increases gluconeogenesis. The inducement of the PEPCK enzymes occurs in the cytosol. By antagonizing insulin actions, cortisol: 1) Decreases synthesis of glycogen, 2) Decreases uptake of glucose by GLUT-4 transporters
In a bid to increase gluconeogenic substrates, glucocorticoids establish a state of catabolism in muscles, inducing the breakdown of peripheral muscle fibers and mobilizing towards the liver for gluconeogenesis.
Glucocorticoids also activate lipase in adipose tissues resulting in availability of free fatty acids for beta-oxidation. These metabolic actions exerted by glucocorticoids clearly explains the mechanism of action of exogenous glucocorticoid medication.
Glucocorticoids facilitate a decrease in muscle mass, thinning of the skin, easy bruising and fragility of the skin. Glucocorticoids are also involved in lipodystrophy and hyperglycemia. However, very little is known of the mechanism of glucocorticoids in lipodystrophy.7
Neurological effects
Some individuals presenting with hypercortisolemia may experience depression. This effect of hypercortisolemia, resulting from glucocorticoid-induced neuronal excitation, may contribute to the pathogenesis of major depressive disorder. Patients with hypercortisolemia may experience bouts of insomnia, slow-wave sleep, and a reduction in REM sleep latency. Individuals in this category also have frequent alterations in electroencephalogram patterns. On the other hand, cortisol insufficiency makes it difficult to carry out mentally-demanding tasks.8
Feedback control of the HPA axis regulates levels of endogenously synthesized glucocorticoids and prevents the drastic effects in bodily functions.
Immune-suppressive and anti-inflammatory functions
Glucocorticoids increases net white blood cell count. The increased white blood cell count is characterized by a decrease in neutrophil migration within the tissue, inhibition of neutrophil apoptosis, promote maturation of white blood cells in the bone marrow, and release into general circulation. Glucocorticoids induce apoptosis of eosinophils and their sequestration in the periphery. Interleukin-2 signal inhibition, impaired release of lymphoid cells, apoptosis of T lymphocyte, NF-kB inhibition, and inhibition of degranulation of mast cells are some of the effects of glucocorticoids in lymphatic tissue. When glucocorticoid levels are increased in the blood, the macrophages of the reticuloendothelial system will be unable to identify antigens and phagocytize them. Regression in lymphoid tissue size is a sequela of these effects.9
Types of Glucocorticoids
Glucocorticoids are a class of corticosteroid hormones that are used clinically for their potent anti-inflammatory and immunosuppressive effects. These hormones have a similar chemical structure, consisting of a tetracyclic steroid nucleus with three six-membered rings and one five-membered ring, and a hydroxyl group at the 17th carbon position.
The various types of glucocorticoids differ in their chemical properties, such as their potency, bioavailability, metabolism, and pharmacokinetics, which can affect their clinical efficacy and safety. Here are some examples of the chemical properties of the commonly encountered glucocorticoids in clinical practice:
- Hydrocortisone: Hydrocortisone, is structurally similar to cortisol, a naturally occurring glucocorticoid. Hydrocortisone has moderate anti-inflammatory and immunosuppressive effects. Hydrocortisone is metabolized by the liver and kidneys to inactive metabolites and has a short half-life of about 90 minutes. Hydrocortisone has a low binding affinity for the glucocorticoid receptor and is less potent than most synthetic glucocorticoids. Hydrocortisone is available in oral, intravenous, topical, and rectal formulations.
- Prednisone: Prednisone is a synthetic glucocorticoid that is derived from cortisol and has greater anti-inflammatory and immunosuppressive effects than hydrocortisone. Prednisone is metabolized by the liver to the active metabolite prednisolone, which has a longer half-life of about 2-3 hours. Prednisone has a higher binding affinity for the glucocorticoid receptor and is more potent than hydrocortisone. Prednisone is available in oral and intravenous formulations.
- Methylprednisolone: Methylprednisolone is a synthetic glucocorticoid that is similar to prednisone but has a methyl group at the 6th carbon position, which increases its potency and bioavailability. Methylprednisolone is metabolized by the liver to inactive metabolites and has a longer half-life of about 18-36 hours. Methylprednisolone has a high binding affinity for the glucocorticoid receptor and is more potent than prednisone. Methylprednisolone is available in oral, intravenous, and intramuscular formulations.
- Dexamethasone: Dexamethasone is a synthetic glucocorticoid that has a fluorine atom at the 9th carbon position, which increases its potency and bioavailability. Dexamethasone is metabolized by the liver to inactive metabolites and has a long half-life of about 36-54 hours. Dexamethasone has a very high binding affinity for the glucocorticoid receptor and is the most potent glucocorticoid. Dexamethasone is available in oral, intravenous, and intramuscular formulations.
| Glucocorticoids | Equivalent Dose (mg) | Glucocorticoid Potency | Mineralocorticoid Potency | Half-life (hrs) | Physiologic Dose |
| Short-Acting | |||||
| Hydrocortisone | 20 | 1 | 1 | 2 | 6 - 12 mg/m2/day |
| Cortisone | 25 | 0.8 | 0.8 | 0.5 | |
| Intermediate-Acting | |||||
| Prednisone | 5 | 4 | 0.3 | 2.5 | 1.5 - 3 mg/m2/day |
| Prednisolone | 5 | 5 | 0.3 | 2 | 1.5 - 3 mg/m2/day |
| Methylprednisolone | 4 | 5 | 0 | 2 | 1.2-2.4 mg/m2/day |
| Long-Acting | |||||
| Dexamethasone | 0.75 | 30 | 0 | 4.5 | 0.2-0.4 mg/m2/day |
| Betamethasone | 0.6 | 25-40 | 0 | 6.5 | |
Related testing & takeaway
Glucocorticoids, such as cortisol, can be measured in plasma or serum using a variety of laboratory tests. The most common method used to test glucocorticoids in plasma is the immunoassay. Immunoassays are based on the principle of specific antibody-antigen interactions. In this case, the assay uses an antibody that binds to a glucocorticoid of interest, such as cortisol. The antibody is labeled with a detectable molecule, such as an enzyme or a fluorescent compound. When a sample containing the glucocorticoid is added to the assay, it competes with a known amount of the labeled antibody for binding to the specific antibody. The amount of labeled antibody that is bound to the specific antibody is inversely proportional to the concentration of the glucocorticoid in the sample.
There are different types of immunoassays used for measuring glucocorticoids in plasma, including radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), and chemiluminescence immunoassay (CLIA). These assays vary in their sensitivity, specificity, and practicality, depending on the purpose and setting of the test. In addition to immunoassays, gas chromatography-mass spectrometry (GC-MS) is another method that can be used to measure glucocorticoids in plasma. GC-MS is a highly sensitive and specific technique that separates and detects individual compounds based on their molecular weight and chemical properties. However, it is more complex and expensive than immunoassays and requires specialized equipment and expertise.
It is important to recall the following points about cortisol testing:
- Cortisol secretion exhibits a circadian rhythm
- There is an increase in cortisol concentration during stress
- Cortisol binding protein level increases in hyper-estrogenic conditions such as hyperthyroidism, pregnancy, diabetes, and in some hematological disorders. These triggers a rise in serum cortisol levels to help maintain balance between the unbound and bound state of cortisol.
- Corticosteroid-binding globulin (CBG) may decrease in hypothyroidism, familial CBG deficiency, and states of protein deficiency such a nephrotic syndrome and severe liver disease. Cortisol levels are also increased by synthetic glucocorticoids, estrogen-based medications, and decreased by phenytoin and androgen.10-15
Finally, dexamethasone is a synthetic glucocorticoid that has a different chemical structure from endogenous cortisol, and it is not typically detectable on standard cortisol immunoassays (unlike hydrocortisone, prednisone, and methylprednisolone).
In fact, dexamethasone is sometimes used in clinical settings to suppress endogenous cortisol production as part of the dexamethasone suppression test (DST) to diagnose certain endocrine disorders. The DST involves administering a low dose of dexamethasone to a patient and measuring the cortisol levels in the blood or urine before and after the dose. The aim of the test is to assess the ability of the HPA axis to respond to the feedback regulation of cortisol. Therefore, in the context of steroid testing, dexamethasone would likely require a different and more specialized method, such as GC-MS or LC-MS/MS, for detection and confirmation.
https://youtu.be/P22rjlgzT7Q
Frequently Asked Questions
Glucocorticoids vs Corticosteroids – is there a difference?
The terms glucocorticoids and corticosteroids are often used interchangeably, but they are not exactly the same. Corticosteroids is a broader term that refers to a class of steroid hormones that are produced by the adrenal cortex, including glucocorticoids, mineralocorticoids, and androgens. Glucocorticoids, on the other hand, specifically refer to the subset of corticosteroids that have a primary function of regulating glucose metabolism, immune response, and stress response, such as cortisol and prednisone.
Therefore, all glucocorticoids are corticosteroids, but not all corticosteroids are glucocorticoids. Mineralocorticoids, such as aldosterone, are another type of corticosteroid that play a role in regulating electrolyte balance and blood pressure. Androgens, such as dehydroepiandrosterone (DHEA), are also corticosteroids that have functions in growth, development, and reproductive physiology. In clinical practice, corticosteroids are often used as a broad term to refer to any type of steroid hormone that has anti-inflammatory or immunosuppressive effects, such as for the treatment of inflammatory and autoimmune conditions. However, depending on the specific condition and purpose of the treatment, different types and doses of corticosteroids may be used, and the effects and side effects may vary.
References
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine reviews. 2011; 32:81–151. [PubMed: 21051590]
- Henley D, Lightman S, Carrell R. Cortisol and CBG – Getting cortisol to the right place at the right time. Pharmacol Ther. 2016; 166:128-35.
- Dammann C, Stapelfeld C, Maser E. Expression and activity of the cortisol-activating enzyme 11β-hydroxysteroid dehydrogenase type 1 is tissue and species-specific. Chem Biol Interact. 2019 25; 303:57-61
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011; 32(1):81-151.
- Russell G, Lightman S. The human stress response. Nat Rev Endocrinol. 2019; 15(9):525-534
- Exton JH. Regulation of gluconeogenesis by glucocorticoids. Monogr Endocrinol. 1979; 12:535-46
- Nieman LK. Cushing’s syndrome: update on signs, symptoms and biochemical screening. Eur J Endocrinol. 2015;173(4):M33-8.
- Daban C, Vieta E, Mackin P, Young AH. Hypothalamic-pituitary-adrenal axis and bipolar disorder. Psychiatr Clin North Am. 2005; 28(2):469-80
- Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011; 335(1):2-13
- Torpy DJ, Bachmann AW, Grice JE, Fitzgerald SP, Phillips PJ, Whitworth JA, Jackson RV. Familial corticosteroid-binding globulin deficiency due to a novel null mutation: association with fatigue and relative hypotension. J Clin Endocrinol Metab. 2001; 86(8):3692-700.
- Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. 2014; 94(12):1816-25.
- Plechner AJ. Cortisol abnormality as a cause of elevated estrogen and immune destabilization: insights for human medicine from a veterinary perspective. Med Hypotheses. 2004; 62(4):575-81.
- Kara C, Ucaktürk A, Aydin OF, Aydin M. Adverse effect of phenytoin on glucocorticoid replacement in a child with adrenal insufficiency. J Pediatr Endocrinol Metab. 2010; 23(9):963-6.
- Gaffey AE, Wirth MM, Hoks RM, Jahn AL, Abercrombie HC. Circulating cortisol levels after exogenous cortisol administration are higher in women using hormonal contraceptives: data from two preliminary studies. 2014; 17(4):314-20.
- Brownlee KK, Moore AW, Hackney AC. Relationship between circulating cortisol and testosterone: influence of physical exercise. J Sports Sci Med. 2005; 4(1):76-83.
Kindly Let Us Know If This Was helpful? Thank You!


