Graves’ disease is an autoimmune thyroid disorder that results in the overproduction of thyroid hormones. This can lead to several symptoms, including anxiety, weight loss, and fatigue. Graves’ disease is the most common cause of hyperthyroidism during pregnancy, and it can pose a serious threat to both mother and child.
Left untreated, Graves’ disease can lead to preterm labor, placental abruption, and stillbirth. Management of hyperthyroidism in pregnancy requires an appreciation of normal thyroid hormone physiology during gestation, the role of anti thyroid medications in management, and mitigating strategies to prevent fetal harm. Treatment of Graves disease during pregnancy is essential to ensure a healthy outcome for both mother and child.
Normal thyroid hormone physiology in pregnancy
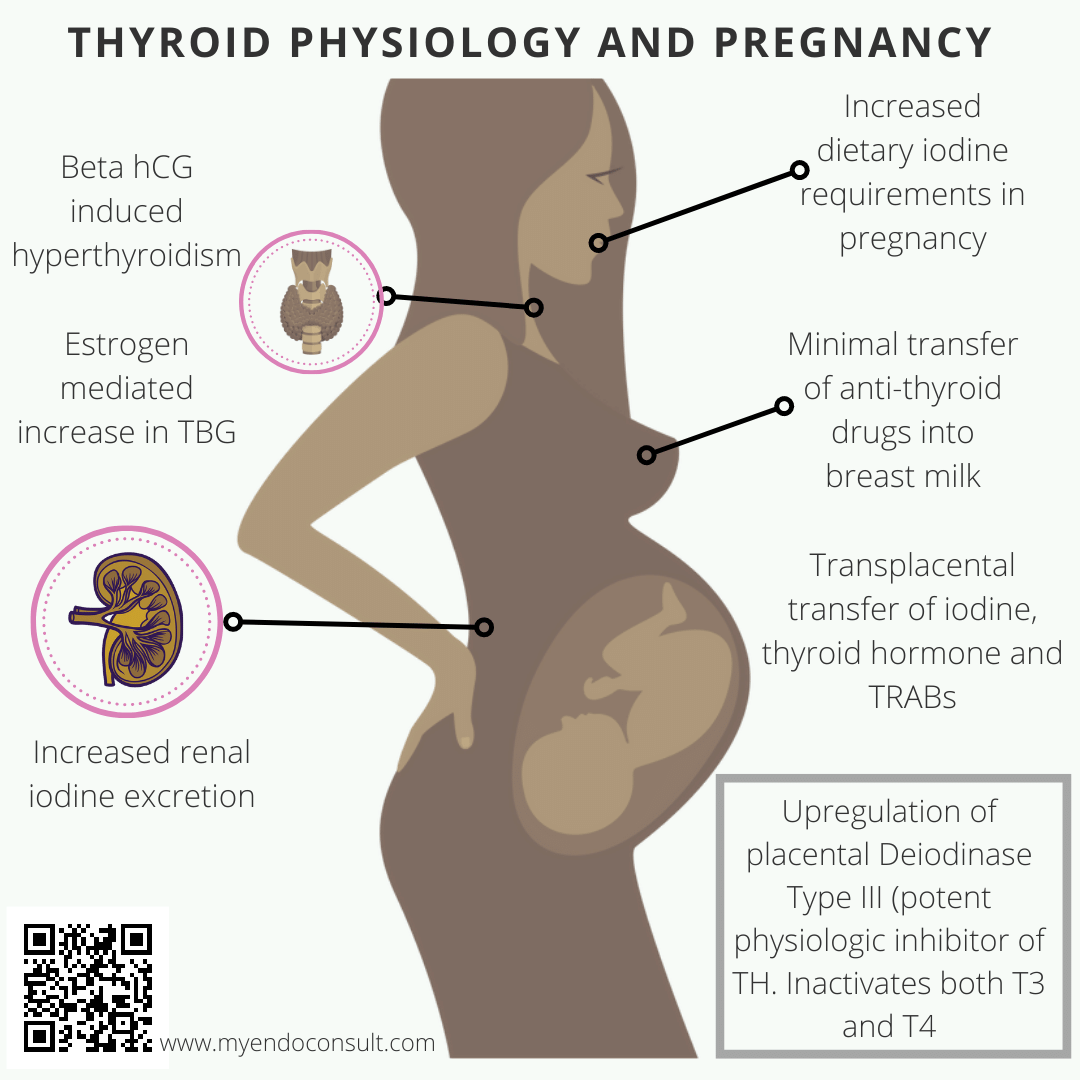
Human chorionic Gonadotropin (hCG) is structurally similar to thyroid-stimulating hormone (thyrotropin) at the alpha subunit. Therefore placental hCG can stimulate the thyroid gland of a pregnant woman leading to productive hyperthyroidism.
Consequently, excess thyroid hormone leads to negative feedback inhibition of endogenous thyrotropin production. For this reason, pregnant women typically have a median TSH level that is comparatively lower than that of their nonpregnant counterparts. This is even more pronounced in the first trimester says, which is when hCG levels tend to peak during pregnancy.
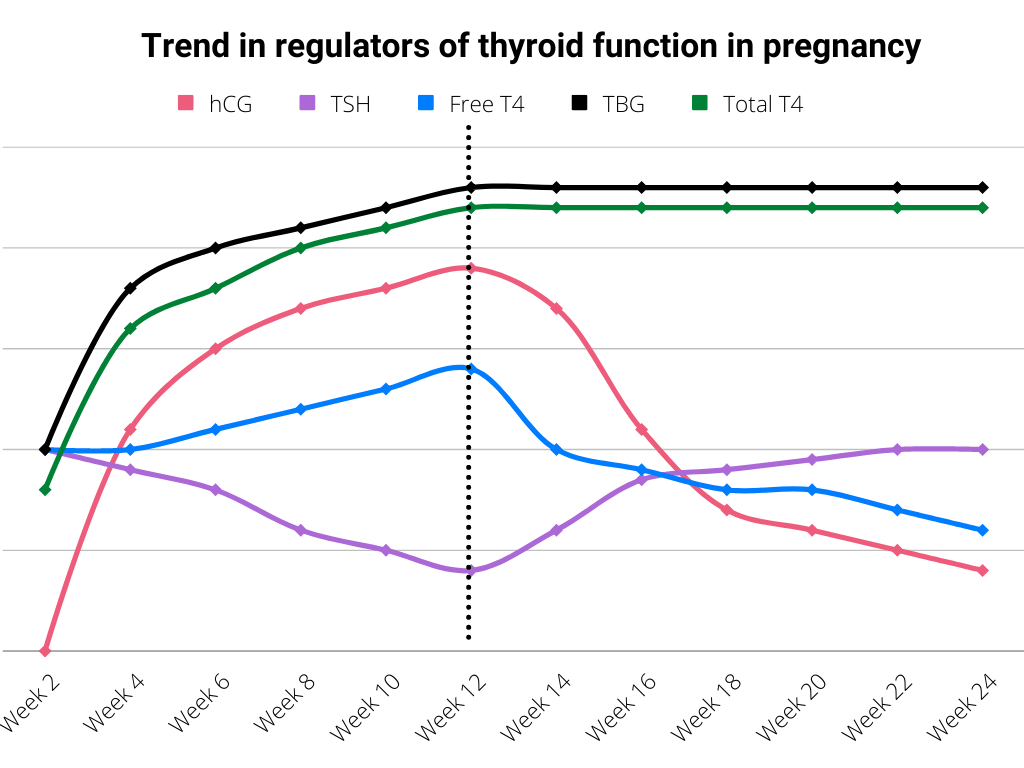
It is essential to note that maternal thyrotropin-releasing hormone plays a critical role in the growth and development of the fetus. Indeed, thyrotropin-releasing hormone and thyroxine (T4) cross the placenta to the fetus during intrauterine life.
Thyroxine (T4) assists in the neurological development of the fetus in the first trimester, up until the fetus’s hypothalamo-pituitary-thyroid axis develops by 12 weeks of gestation.
Deiodinase type III (D3) enzyme is typically expressed by the placenta and regulates the availability of thyroxine to the developing baby. Consequently, in the setting of maternal hyperthyroidism, this enzyme will prevent overexposure of the fetus to high levels of thyroid hormone, by converting both T3 and T4 into their inactive metabolites.. This makes D3 the most potent physiologic inactivator of thyroid hormone.
Also, iodine, critical for the formation of thyroid hormone, crosses the placenta freely since there is a ubiquitous expression of sodium iodide symporters (NIS) by syncytiotrophoblasts. TSH receptor antibodies (thyroid-stimulating immunoglobulin and thyrotropin binding inhibitory immunoglobulin) typically cross the placenta and may negatively impact fetal thyroid function.
Symptoms of Graves Disease during pregnancy
Graves disease can lead to a number of symptoms, including weight loss, anxiety, tremors, and increased heart rate. Graves’ disease can also affect the eyes, causing bulging and inflammation. In pregnant women, Graves’ disease can lead to premature birth, low birth weight, and miscarriages.
Physical Exam for Graves disease in pregnancy
In Graves disease, physical exam findings may include goiter, tachycardia, tremulousness of the extremities, and ophthalmopathy. During pregnancy, these findings may be more pronounced. Additionally, pregnant women with Graves disease may have an increased risk for preeclampsia (high blood pressure) and other complications.
Preconception planning for Graves’ disease
A woman with Graves’ disease should be clinically and biochemically euthyroid before proceeding with pregnancy. Pregnant women who are thyrotoxic on anti thyroid drugs or euthyroid on relatively high doses of antithyroid drugs should be considered for some form of definitive therapy before considering pregnancy.
Depending on the definitive therapy chosen, the “time-to-pregnancy” can vary significantly. For example, for women who choose to have radioactive iodine ablation, contraception is required for a minimum of six months to prevent exposure of the fetus to the effects of radiation.
Women who opt for total thyroidectomy may proceed with pregnancy after normalizing their thyroid status.
The clinical course of Graves’ disease in pregnancy
As was previously mentioned, expectant mothers may normally develop mild hyperthyroidism during pregnancy. This is more likely in the first trimester due to beta hCG levels reaching their peak during this phase of pregnancy.
This effect accounts for both transient hCG-mediated hyperthyroidism and hyperemesis gravidarum of pregnancy. Both conditions are self-limiting and resolve by 14 to 18 weeks of gestation with minimal complications.
In addition, women with Graves’ disease may experience a significant exacerbation of hyperthyroid symptoms during the first trimester (peak effect of hCG), although there is an expected improvement during the second and third trimesters.
The table below summarizes the clinical course of Graves’ disease during pregnancy.
| Stage of pregnancy | Clinical course | Mechanism |
| First Trimester | Aggravation of graves hyperthyroidism | HCG-mediated thyroid hormone production |
| Second and third trimester | Significant improvement in hyperthyroidism | An increase in thyroxine-binding globulin (data were estrogen production) leads to a reduction in free thyroid hormone. Suppression of thyroid autoimmunity by estradiol, progesterone, and cortisol. |
| Postdelivery | Worsening thyroid function | Reactivation of thyroid autoimmunity in the absence of placental steroids |
Management of Graves’ disease in pregnancy
The goal of management is to prevent exposure of the fetus to an excessively high amount of maternally derived thyroid hormone while maintaining near-optimal thyroid hormone levels in the expectant mother.
For this reason, women with Graves’ disease should be borderline hyperthyroid in order to reduce the risk of fetal harm. Indeed, antithyroid medications should not be initiated if the patient has subclinical, asymptomatic, or mild overt hyperthyroidism.
Patients should generally have their thyroid function test evaluated every 4 to 6 weeks. For patients requiring treatment (moderate to severe overt hyperthyroidism), propylthiouracil is recommended in the first trimester due to the risk of choanal atresia (birth defects) associated with methimazole.
During the second and third trimesters, patients should be switched from propylthiouracil to methimazole due to the high risk of liver injury associated with the former.
Beta-blockers (atenolol) help control adrenergic symptoms, although they should be discontinued after an improvement in free thyroid hormone levels. While beta-blockers are generally considered safe, there is some concern that they may be associated with adverse effects in pregnant women. In particular, beta-blockers have been linked to an increased risk of miscarriage and intrauterine growth restriction.
Thyrotropin receptor antibodies (TRAB) should be measured in the third trimester in all patients with Graves’ disease. With TRAB titers at least three times the upper limit of normal, being highly predictive of neonatal hyperthyroidism. This test is important because TRABs can cross the placenta (during separation) and stimulate the thyroid of the neonate after delivery.
Safety of breastfeeding while on anti-thyroid medications
Mothers with Graves disease who are taking methimazole or propylthiouracil (PTU) can breastfeed if they have their infant’s thyroid function checked and maintain their own thyroid function within the normal range.
Traditionally, anti-thyroid drugs have been discouraged for breastfeeding mothers. Although both propylthiouracil and methimazole can be detected in milk, the concentrations are quite minimal, leaving little to no fetal harm.
Indeed, studies have shown that the milk to plasma ratio of propylthiouracil is 0.1, with less than 3% of the weight-adjusted dose of propylthiouracil being present in the feeding. For mothers on methimazole, the maximum amount of weight-adjusted methimazole present in breast milk is about 12%. Indeed, it has been shown that in patients treated with 20 mg of methimazole per day, no demonstrable clinical harm occurs in the infant.
Because both methimazole and PTU can cross into breast milk, mother and infant should be monitored closely for signs and symptoms of hypothyroidism or hyperthyroidism. If either condition develops, the infant should be evaluated by a pediatrician and the mother’s dose of methimazole or PTU should be adjusted accordingly.
Treatment of Graves’ disease with either surgery or radioactive iodine does not lead to an amelioration of thyroid-related autoimmunity. Indeed, these patients may still have high levels of circulating thyroid-stimulating immunoglobulins, which can not lead to hyperthyroidism in the mother but can still cross the placenta and lead to neonatal thyrotoxicosis.
References
Hamburger JI. Diagnosis and management of Graves’ disease in pregnancy. Thyroid. 1992 Fall;2(3):219-24.
Patil-Sisodia K, Mestman JH. Graves hyperthyroidism and pregnancy: a clinical update. Endocr Pract. 2010 Jan-Feb;16(1):118-29.
De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012 Aug;97(8):2543-65.
Kindly Let Us Know If This Was helpful? Thank You!


