Causes of Hyperthyroidism
Hyperthyroidism, also known as thyrotoxicosis, is often a result of an overactive thyroid gland, which is commonly associated with an autoimmune condition called Graves' disease. In patients with this condition, the bloodstream carries specific thyroid-stimulating immunoglobulins (TSIs) that attach to the TSH receptors on the thyroid's follicular cells, stimulating them to produce thyroid hormone just like natural TSH does.
Autoantibodies against thyroglobulin and thyroid peroxidase may also be present. Graves' disease is more prevalent in women aged 40 to 50, being five to eight times more common than in men, and it frequently co-occurs with other autoimmune disorders such as myasthenia gravis, Addison's disease, and pernicious anemia.
Key symptoms of Graves' disease include:
- High basal metabolic rate—this leads to a general increase in body metabolism; more heat production causes heat intolerance, excessive sweating, and warm skin.
- Weight loss despite normal or increased appetite as a result of muscle wasting; other symptoms can include diarrhea and menstrual irregularities.
- Rapid heart rate, trembling, palpitations, and other types of fast heart rhythms (atrial fibrillation in elderly people), and high blood pressure. These symptoms are often due to increased thyroid hormone secretion, which enhances the responses of the sympathetic nervous system, as a result of an increase in the number and affinity of β-adrenergic receptors.
- Psychological symptoms include restlessness, anxiety, nervousness, irritability, hyperexcitability, and emotional instability.
- Changes in eye appearance—in about half of the patients, the eyelids may retract and eyeballs may protrude (known as exophthalmos or proptosis) due to swelling of the muscles surrounding the eyes. This is caused by the infiltration of lymphocytes and the deposition of mucopolysaccharides and fluid in the soft tissues around the eyes.
- Diffuse toxic goiter, which is a symmetrical enlargement of the thyroid gland.
- Elevated levels of thyroid hormones (T3 and T4) in the bloodstream, while the serum TSH level is low or undetectable due to excessive negative feedback effects of the high T3/T4 hormones on the anterior pituitary gland.
Toxic Multinodular Goiter. This condition is often seen in older patients due to the growth of several hyperactive thyroid nodules. Initially, the patient may exhibit normal thyroid hormone levels, but these nodules start operating autonomously over time, resulting in mild hyperthyroidism without exophthalmos. As relapses are frequent after antithyroid drug treatment, the standard treatment generally involves surgery or radioactive iodine. The root cause of this condition remains unknown.
Solitary Toxic Nodule. This situation can arise in patients of any age when a single overactive thyroid nodule (such as a benign autonomous follicular adenoma not controlled by the pituitary) secretes excessive thyroid hormones. Patients usually exhibit mild hyperthyroid symptoms and might be treated with radioiodine or partial thyroidectomy.
Subacute Thyroiditis (De Quervain's Thyroiditis). This is a relatively rare, self-limiting inflammation of the thyroid, likely caused by a virus, which can occur after an upper respiratory tract infection. Depending on the severity of the inflammation, patients may experience thyroid swelling, tenderness, and pain while swallowing. Hyperthyroid symptoms like weight loss, excessive sweating, irritability, rapid heart rate, and tremors might also appear due to the inflammatory release of preformed thyroid hormone. The treatment involves oral pain relievers, corticosteroids for thyroid pain and inflammation, and propranolol for hyperthyroid cardiac effects. A temporary phase of mild hypothyroidism might follow the condition.
Thyrotoxic Crisis (Thyroid Storm). This is an uncommon but severe medical emergency triggered by a sudden release of large quantities of thyroid hormone in a hyperthyroid patient otherwise under control following sudden stress, infection, or after surgery/radioiodine treatment in unprepared patients. Symptoms include extreme restlessness, confusion, abdominal pain, rapid heart rate (possibly leading to heart failure), and fever. Immediate treatment involves propranolol (administered via slow intravenous infusion), antithyroid drugs, and aqueous iodine (either orally or via a nasogastric tube if necessary) alongside corticosteroids, antibiotics, and intravenous fluids/electrolytes.
Secondary hyperthyroidism can also develop from the following scenarios:
- Deliberate consumption of excessive amounts of thyroid hormone in an effort to lose weight (Thyrotoxicosis factitia).
- A pituitary adenoma that produces TSH (rare).
- An ovarian teratoma with thyroid components (Struma ovarii).
- Metastatic thyroid carcinoma (of the follicular type).
- Treatment with the cardiac anti-arrhythmic drug amiodarone.
The TRH test can be an effective tool in diagnosing hyperthyroidism. In this test, a dose of 400 μg of TRH is administered intravenously, and the plasma levels of TSH are measured both before and approximately 20-30 minutes after the injection to examine the anterior pituitary's appropriate response. In patients with primary hyperthyroidism, the anticipated increase in plasma TSH, typically between 5-30 mU/l, is suppressed due to the ongoing negative feedback inhibition of the pituitary by high levels of thyroid hormones. For some patients, the injection of TRH may result in a temporary rise in blood pressure, increased heart rate, or bronchospasm.
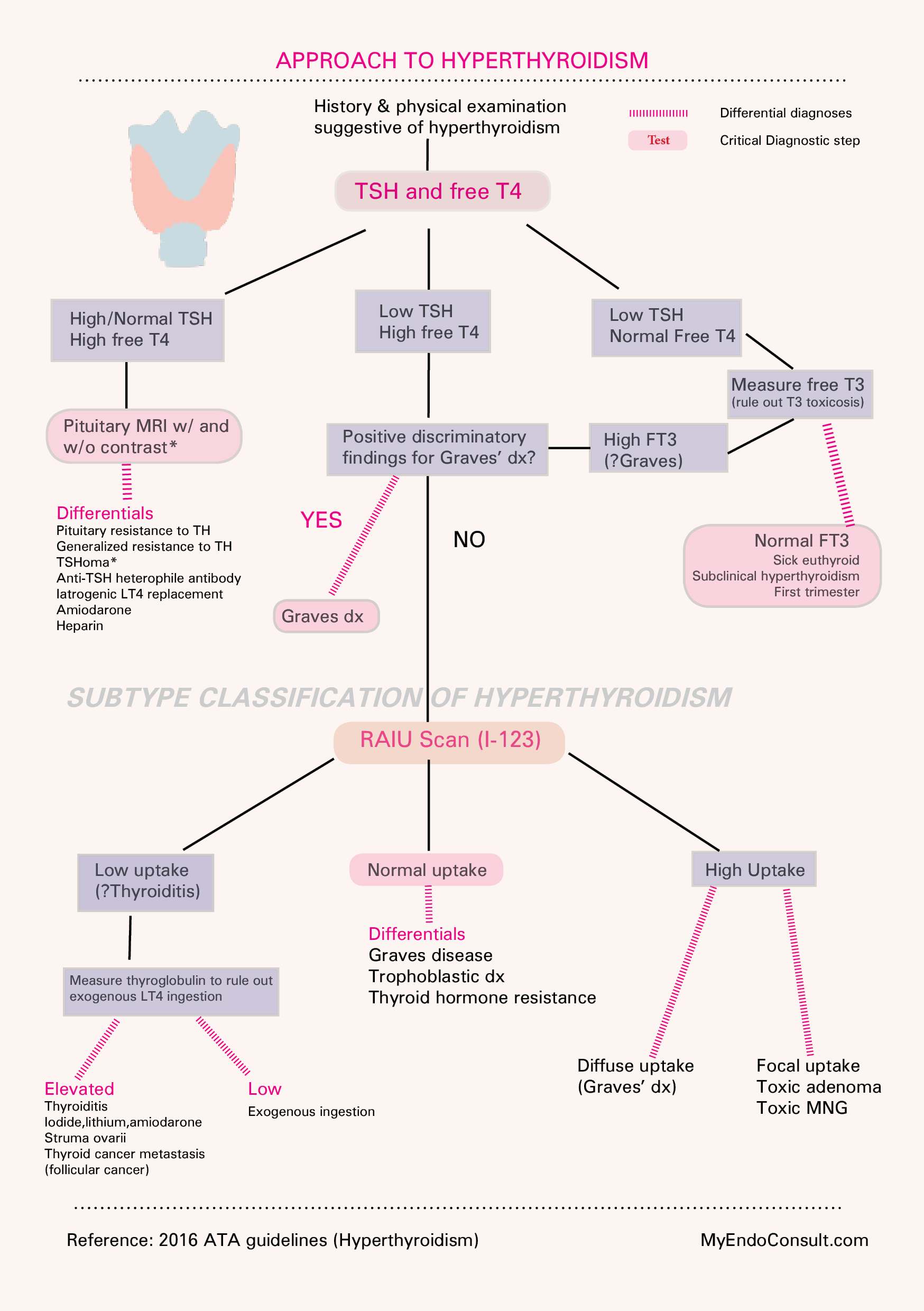
Algorithm for Evaluating Hyperthyroidism
Kindly Let Us Know If This Was helpful? Thank You!