Estrogens are a group of steroid hormones that play an essential role in the female reproductive system, regulating menstruation, pregnancy, and other processes. While they are produced in higher levels in females, estrogens also exist in males and have various roles in both sexes.
- Estrone (E1): This type of estrogen is primarily produced in the ovaries and adipose tissue, especially during menopause. Estrone is weaker than the other estrogens and is the primary estrogen found in women after menopause. It’s made from androstenedione, a hormone produced in the adrenal glands and the ovaries.
- Estradiol (E2): Estradiol is the most potent and prevalent estrogen in reproductive-aged women. It’s produced primarily in the ovaries but also in the adrenal glands and the placenta during pregnancy. Estradiol regulates the menstrual cycle and prepares the body for pregnancy by thickening the lining of the uterus. It also has several other roles in the body, influencing bone density, skin health, mood, and cardiovascular health, among other things.
- Estriol (E3): This estrogen is produced in significant amounts only during pregnancy. The placenta, an organ that develops in the uterus during pregnancy, produces estriol from precursors supplied by both the mother and the fetus. Estriol increases as pregnancy progresses and reaches its peak at term. It’s thought to promote the growth of the uterus and prepare the mother’s body for delivery. It is also the primary estrogen produced during pregnancy.
In addition to these naturally occurring estrogens, synthetic and semi-synthetic estrogens, such as ethinylestradiol, are used in various pharmaceutical applications, including hormonal contraception.
The balance and interplay of these different forms of estrogen can influence a wide array of physiological processes and conditions, ranging from bone health to cardiovascular function, reproductive health, and even certain types of cancer. Estrogens act by binding to specific estrogen receptors within cells, which triggers changes in gene expression and cellular function.
Mechanism of action of Estrogens
Estrogens are steroid hormones that function by binding to and activating specific receptors inside target cells – estrogen receptors (ERs). These receptors are proteins found in various tissues in the body, including those of the female reproductive system, the breast, bone, the liver, and the cardiovascular system. Two primary forms of estrogen receptors exist, ER-alpha and ER-beta.
The mechanism of estrogen action involves the following steps:
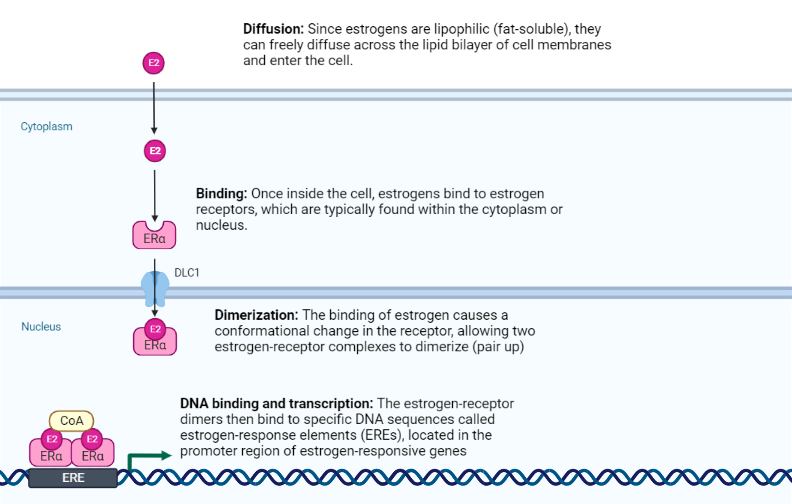
- Diffusion: Since estrogens are lipophilic (fat-soluble), they can freely diffuse across the lipid bilayer of cell membranes and enter the cell.
- Binding: Once inside the cell, estrogens bind to estrogen receptors, which are typically found within the cytoplasm or nucleus.
- Dimerization: The binding of estrogen causes a conformational change in the receptor, allowing two estrogen-receptor complexes to dimerize (pair up).
- DNA binding and transcription: The estrogen-receptor dimers then bind to specific DNA sequences called estrogen-response elements (EREs), located in the promoter region of estrogen-responsive genes. This binding allows for the recruitment of other proteins necessary for the transcription of DNA into messenger RNA (mRNA).
- Translation: The mRNA then leaves the nucleus and is translated into new proteins in the cytoplasm, a process that results in the specific effects seen with estrogen activity.
This whole process is referred to as the genomic mechanism of estrogen action. It generally takes hours to days due to the time required for transcription and translation. However, estrogens can also act through non-genomic mechanisms, which involve interactions with cell membrane-bound estrogen receptors and intracellular signaling pathways, causing more immediate cell responses.
Both genomic and non-genomic actions contribute to the many physiological effects of estrogens, including the development and maintenance of the female reproductive system, secondary sexual characteristics, bone health, cardiovascular health, and modulation of various metabolic processes.
Physiologic effects of estrogen
| Physiologic role | Mechanism |
| Mammary gland | Estrogen promotes the proliferation of mammary tissue during puberty. Also responsible for cyclical proliferation and involution during the menstrual cycle (reproductive years) |
| Bone | It promotes initial pubertal growth spurt and paradoxically limits final skeletal height by inducing ossification (closure) of the epiphyseal growth plate. The effects of estrogen on bone resorption |
| Lipid metabolism | Estrogen lowers triacylglycerols by increasing the expression of apolipoproteins required for lipid transport |
| Glucose metabolism | Estrogen exerts a myriad of effects on lipid metabolism by, Increasing peripheral tissue sensitivity by increasing the expression of glucose transporter 4 (GLUT4) – required for glucose uptake by skeletal muscle and adipose tissue. Reducing inflammation in adipose tissue (thus, insulin resistance) |
Metabolism of Estrogens
Estrogens, the primary female sex hormones, undergo a complex metabolic process to maintain homeostasis and exert their functions in the body. Here is a brief overview of the metabolic pathways involved in the various forms of estrogen:
1. Synthesis: Estrogens are synthesized from cholesterol in a multi-step process. The process begins in the adrenal glands and ovaries, where cholesterol is converted to pregnenolone and then to progesterone. The progesterone is then converted into androgens (like androstenedione and testosterone), which are the immediate precursors of estrogens.
2. Conversion: The conversion of androgens to estrogens is catalyzed by the enzyme aromatase. This enzyme converts androstenedione to estrone and testosterone to estradiol. This conversion can occur not only in the ovaries and adrenal glands but also in other tissues, such as adipose tissue, the brain, and the placenta.
3. Distribution: Once formed, estrogens travel through the bloodstream, mostly bound to sex hormone-binding globulin (SHBG) and albumin. A small portion remains unbound or “free” and is biologically active.
4. Receptor Binding and Action: Estrogens exert their effects by binding to estrogen receptors (ERs), which can be found throughout the body. These receptors are part of the nuclear receptor family of intracellular receptors and can directly modulate gene expression when activated by estrogen.
5. Metabolism: Estrogens undergo further metabolism in the liver where they are converted into less active compounds. This metabolism mainly involves the addition of hydroxyl groups (hydroxylation) and the addition of a molecule called glucuronide (glucuronidation), which makes the hormones more water-soluble and easier to excrete. The metabolites are then excreted in the bile into the digestive tract, and some are reabsorbed back into circulation.
6. Excretion: Eventually, the water-soluble metabolites are eliminated from the body through the urine and feces. The balance between synthesis, metabolism, and excretion determines the levels of estrogens in the body.
Estrogen metabolism is a complex process that can be influenced by various factors, including age, diet, genetic factors, and environmental exposure. Dysregulation in estrogen metabolism can lead to conditions such as estrogen dominance or deficiency, which are associated with various health issues like menstrual disorders, menopausal symptoms, and an increased risk for certain types of cancer.
Clinical Applications of Estrogens
In the clinical realm, the utilization of estrogens, including 17β-estradiol, oestrone, estriol, ethinylestradiol, mestranol, dienestrol, and stilboestrol, has been proven beneficial for several conditions. Hormone replacement therapy (HRT) remains a common application, particularly in young women with primary hypogonadism or other hypo ovarian disorders, and in alleviating menopausal symptoms.
Menopausal symptoms such as hot flushes, night sweating, palpitations, headaches, and vaginal dryness and atrophy are linked to the diminished natural production of estrogens by the ovaries. Women who have undergone an oophorectomy or hysterectomy due to cancer or severe endometriosis may also experience these symptoms prematurely.
Estrogens play a pivotal role in mitigating the effects of parathyroid hormone on bone, thus preventing the onset of post-menopausal osteoporosis and reducing bone fracture rates in the long term. Estrogens also confer protection against coronary heart disease in postmenopausal women, primarily by favorably modulating lipid metabolism and enhancing arterial compliance. This benefit could extend to women already diagnosed with cardiovascular disease.
For women with an intact uterus, combined estrogen/progestogen therapy is typically advised to decrease the risk of endometrial carcinoma. However, for those who have undergone a hysterectomy, estrogen-alone HRT is suitable. In most cases, ‘natural’ estrogens like oestradiol, oestrone, or oestriol are preferred over potent synthetic derivatives, and clinicians strive to administer the lowest effective maintenance dose whenever feasible.
Clinical Uses of Estrogens
Estrogens come in various forms and are commonly used for different clinical purposes. Let’s delve into some of the prevalent types of estrogen-only preparations:
- Oral ‘natural’ estrogen therapy: Daily oral doses of tablet preparations containing oestradiol valerate, piperazine oestrone sulphate, estriol, or conjugated equine estrogens are frequently used. These are usually provided in 28-tablet blister strips and are taken on a continual basis.
- Transdermal therapy: This method is particularly useful for patients who have difficulty tolerating oral therapy or those who struggle with medication compliance. The advantage here lies in avoiding first-pass metabolism of the hormone by the liver. However, this option tends to be more expensive and may result in local skin irritation. Currently, available self-adhesive estrogen-only patches containing 17β-oestradiol can deliver between 25-100 μg hormone/24 hours. These patches are placed on the lower trunk and replaced every 3-4 days. Also, newer ‘7-day’ oestradiol patches are now available, delivering around 50 μg hormone/24 hours.
An alternative form of transdermal therapy is an estrogen-containing gel, which can be applied to the arms, shoulders, or inner thighs, but not the breasts or vaginal region. This gel could be useful for women with skin allergies to patches or those who prefer not to use oral therapy. In cases where the patient has an intact uterus, additional oral progestogen tablets may be co-prescribed.
- Implant therapy: This treatment is a potential alternative to oral or transdermal therapy. In this case, 17β-oestradiol is inserted subcutaneously into the buttocks or abdomen under clinical supervision. This process is repeated every 4-8 months as necessary. If the uterus is intact, an oral progestogen is also taken for 10-14 days per cycle.
- Vaginal therapy: Estrogens such as 17β-oestradiol, oestriol, conjugated equine estrogens, or dienestrol can also be applied topically in the form of creams, vaginal pessaries, or modified-release tablets. These are used for the short-term treatment of atrophic vaginal symptoms.
A novel form of estrogen therapy has emerged in the form of an impregnated silastic vaginal ring, commercially known as Estring. This device is designed to consistently release approximately 7.5 mg of 17β-oestradiol hemihydrate directly into the vagina every 24 hours. The systemic absorption of oestradiol is minimal, ensuring the effects remain localized.
The vaginal ring is designed to be worn continuously and requires replacement every three months. The therapy can be sustained for up to two years, depending on the patient’s needs and response. It’s essential that patients under this form of therapy undergo regular check-ups every three to six months, allowing healthcare professionals to evaluate the ongoing necessity and effectiveness of the treatment.
Comparison of oral and transdermal estrogens
| Effect(s) | Oral estrogen | Transdermal estrogen |
| First metabolism in the liver | Yes | No |
| Low-density lipoprotein | ↓↓ | ↓ |
| High-density lipoprotein | ↑↑ | ↑ |
| Triglycerides | ↑↑ | → |
| Coagulation factors | ↑↑ | → |
Estrogen-based therapies with efficacy in vasomotor symptoms
| Estrogen-based treatment (route) | Standard dose (Low dose) |
| Conjugated estrogen (oral) | 0.625mg/d (0.3 to 0.45mg/d) |
| Micronized estradiol-17β (oral) | 1mg/d (0.5mg/d) |
| Estradiol-17β (transdermal) | 0.0375 to 0.05mg/d (0.025mg/d) |
| Conjugated estrogen + bazedoxifenea (oral) | 0.45mg/d + 20mg/d |
Estrogen Antagonists and Aromatase Inhibitors in Medical Treatments
In the wide arena of medical treatments, some specific compounds have shown tremendous promise in addressing health issues like infertility and hormone-dependent tumors. Among these are the estrogen antagonists like clomiphene and tamoxifen, as well as a class of drugs known as aromatase inhibitors, exemplified by aminoglutethimide.
Let’s delve into the specifics of these influential compounds and their clinical use.
Estrogen Antagonists
Estrogen antagonists like clomiphene essentially compete with estrogens for cytoplasmic estrogen receptors. Interestingly, these antagonists exhibit negligible inherent estrogenic activity. Consequently, they inhibit the standard hormonal negative feedback on the hypothalamus and anterior pituitary. The result is an increase in the secretion of gonadotropin-releasing hormone (GnRH) and luteinizing hormone/follicle-stimulating hormone (LH/FSH). Provided the hypothalamus and pituitary are operational, clomiphene serves to stimulate ovulation in infertile women. It’s worth noting, however, that this could lead to hyperovulation and multiple pregnancies.

Tamoxifen operates in a similar manner. It’s employed to manage anovulatory infertility and has emerged as a preferred treatment for advanced estrogen-dependent breast tumors. Another estrogen antagonist, toremifene, is also used in the same context. The potential preventive role of tamoxifen in high-risk women (those with a positive family history) against breast tumor development is currently under scrutiny in the United States.
Like all pharmaceutical compounds, these anti-estrogens carry certain side effects such as hot flushes, vaginal bleeding, abdominal discomfort, dizziness, and visual disturbances. Furthermore, recent studies point to a modest yet significant risk of endometrial cancer following long-term tamoxifen treatment (more than two years).
Aromatase Inhibitors
In the realm of breast cancer management, aminoglutethimide, a potent aromatase enzyme inhibitor, comes into play. This compound inhibits several cytochrome P-450-mediated steroid hydroxylation steps, including the conversion of cholesterol to pregnenolone in the adrenal glands and the peripheral aromatization of androgens to estrogens in fat, muscle, and liver cells.
Aminoglutethimide finds use in treating advanced estrogen-dependent breast cancer in post-menopausal or oophorectomized women. It is particularly effective for patients who have developed resistance to tamoxifen. Additionally, it serves as a palliative treatment for advanced prostatic carcinoma in men.
To mitigate possible side effects like drowsiness, dermatitis, and blood disorders, modern treatment regimens incorporate lower daily doses of the inhibitor (250-500 mg), combined with a replacement dose of a corticosteroid such as cortisone. This therapeutic approach, often termed ‘medical adrenalectomy’, serves as a substitute for bilateral surgical adrenalectomy.
In conclusion, these compounds, their mechanisms, and their therapeutic uses underscore the advances made in the medical treatment of hormone-related health issues
Kindly Let Us Know If This Was helpful? Thank You!


