Overview And Diagnosis of Prolactinomas
- Hyperprolactinaemia refers to elevated levels of prolactin, the only anterior pituitary hormone usually inhibited by the hypothalamus.
- Disconnection of the pituitary gland from the hypothalamus due to a growing tumor can result in a rise in circulating prolactin levels as the lactotrophs are no longer inhibited by dopamine.
- High levels of prolactin can cause galactorrhoea (milk flow not related to nursing) in the estrogen-primed breast and can also suppress the gonadotrophin-releasing hormone (GnRH), leading to amenorrhea (absence of menstrual periods).
- Other causes of hyperprolactinemia can include administration of dopamine antagonist drugs used for conditions like travel sickness or psychiatric diseases such as schizophrenia.
- Prolactinomas, tumors that overproduce prolactin, are a significant cause of hyperprolactinaemia.
Treatment for Prolactinoma
- Dopaminergic agonists are the first line for treatment of prolactinomas.
- Either bromocriptine or cabergoline can be used as treatments.
- If hyperprolactinemia suppresses gonadotrophin production, gonadotrophin axis recovery after DA therapy can occur so long as gonadotrophs aren't affected by mass effect from prolactin secreting tumor.
- If gonadotrophs are profoundly affected by the mass effect of a prolactin secreting tumor, treatment with estrogen or testosterone might be necessary, as their function may not be resotred after normalising prolactin levels.
Dopaminergic agonists
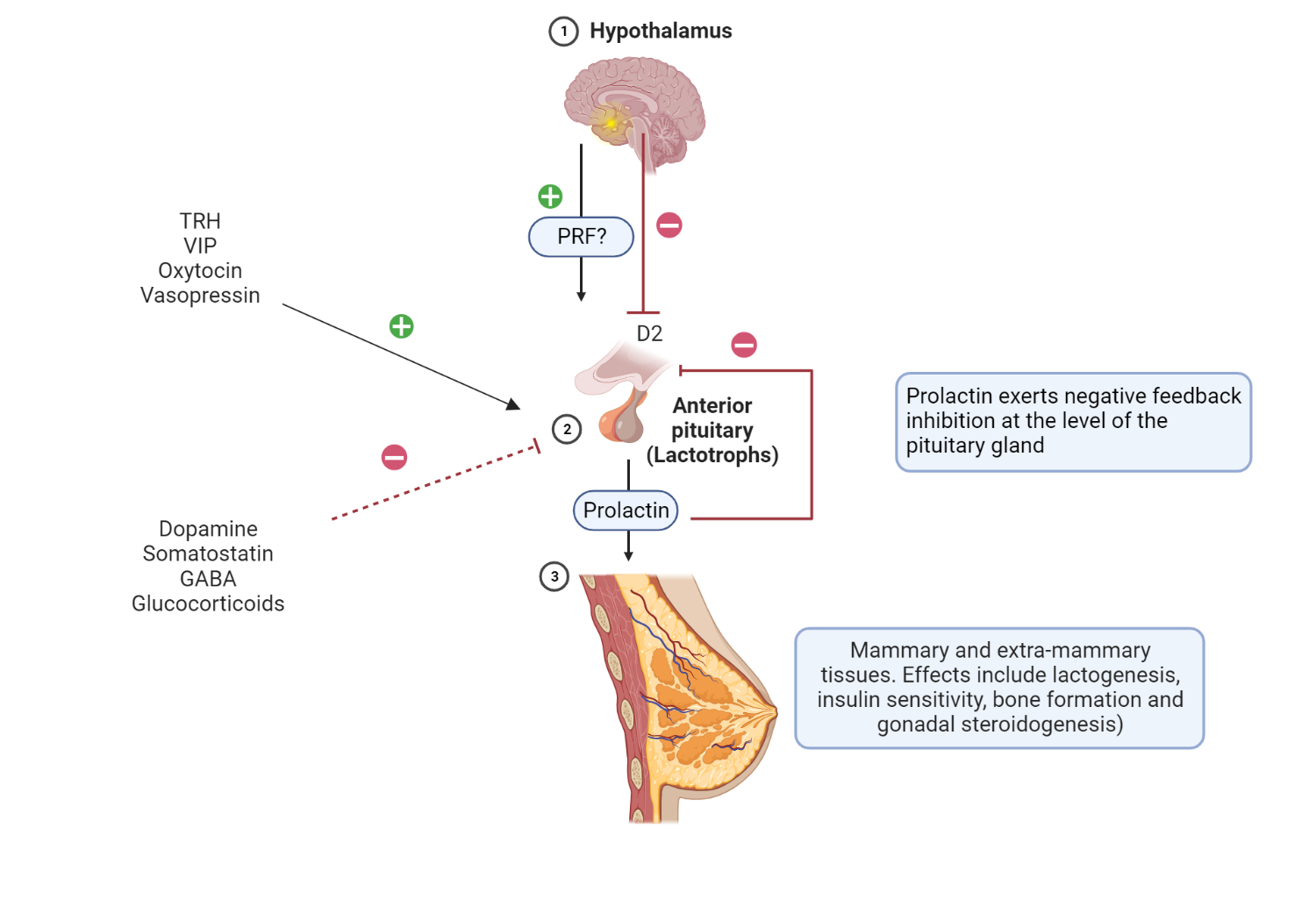
Schematic representation of the hypothalamic-pituitary-mammary axis.
PRF is a putative (unconfirmed) hormone involved in stimulation of PRL release by lactotrophs in the anterior pituitary gland. Hypothalamic dopamine affects prolactin release by binding and activating D2 receptors present in lactotrophs. Stimulatory and inhibitory factors involved in the regulation of PRL release are represented by + and – signs. Extramammary tissues include gonadal steroidogenesis, skeletal growth, and glucose metabolism, to mention a few.
Although classically associated with mammary glands, prolactin has several extramammary effects due to the presence of prolactin receptors in various tissues. There are PRL receptors (PRL-R) in pancreatic beta cells (glucose-mediated insulin release), adipose tissue (thermoregulation), and hematopoietic cells (activation of T cells), to name a few[1,2].
Dopaminergic agonists (cabergoline and bromocriptine), synthetic derivatives of ergots, bind to D2 receptors on the surface of pituitary lactotroph tumors and induce dopamine-mediated inhibition of prolactin synthesis and secretion. Dopaminergic agonists also exhibit "tumoricidal properties" by promoting programmed cell death of lactotroph tumors through complex intracellular pathways that involve estrogen and neuronal dopamine transporters[3].
Patients who do not experience normalization of serum PRL or a 50% reduction in tumor size are classified as resistant to dopaminergic agonists. However, no conventionally accepted dose or duration of exposure to DA is required to diagnose a patient as resistant to DA resistant [4,5].
Predictors of an inadequate response to dopaminergic agonists include male sex, macroprolactinoma, tumor characteristics (cystic or hemorrhagic), prolonged latency to euprolactinemia, and high PRL at baseline[6].
For women in the reproductive age group planning a pregnancy, the use of bromocriptine is a safer therapeutic option compared to cabergoline. This is due to the availability of more safety data for the former compared to the latter[7].
Side effects of DA include nausea, vomiting, headaches, postural hypotension, psychotropic side effects (hallucinations, psychosis) and nasal congestion[8,9]. The dictum "start low and go slow" helps mitigate the side effects of DA. Cabergoline has a longer half-life, a higher affinity for D2R, and a relatively tolerable side effect profile compared to bromocriptine[10].
It is reasonable to screen for psychiatric disorders before initiating dopaminergic agonists. Compulsive gambling has been associated with cabergoline use; it is reasonable to alert patients of this potential side effect[11].
Is there a role for somatostatin analogs in the treatment of prolactinomas?
Dopaminergic agonist-resistant prolactinomas are usually treated with surgical debulking, radiation therapy, or temozolomide[22]. As you may recall, somatostatin inhibits prolactin release[23]. Prolactinomas co-express SSR1 and SSR5 predominantly. The low expression of SSR2 receptors by prolactinomas is responsible for their suboptimal response to octreotide. Pasireotide, being a multireceptor SSA, has an affinity for both SSR1 and SSR5 receptors, making it a viable option in the management of prolactinoma)[24]. There are reports of prolactinomas that have responded to pasireotide[22,25,26].
Temozolomide
Temozolomide (TMZ) is an alkylating chemotherapeutic agent that promotes the methylation of specific residues of guanine (position O-6) and purine (positions N3 and N7) in DNA. This introduces breaks in the DNA of rapidly growing cells, such as lactotrophic tumors, leading to their apoptosis (programmed cell death)[12].
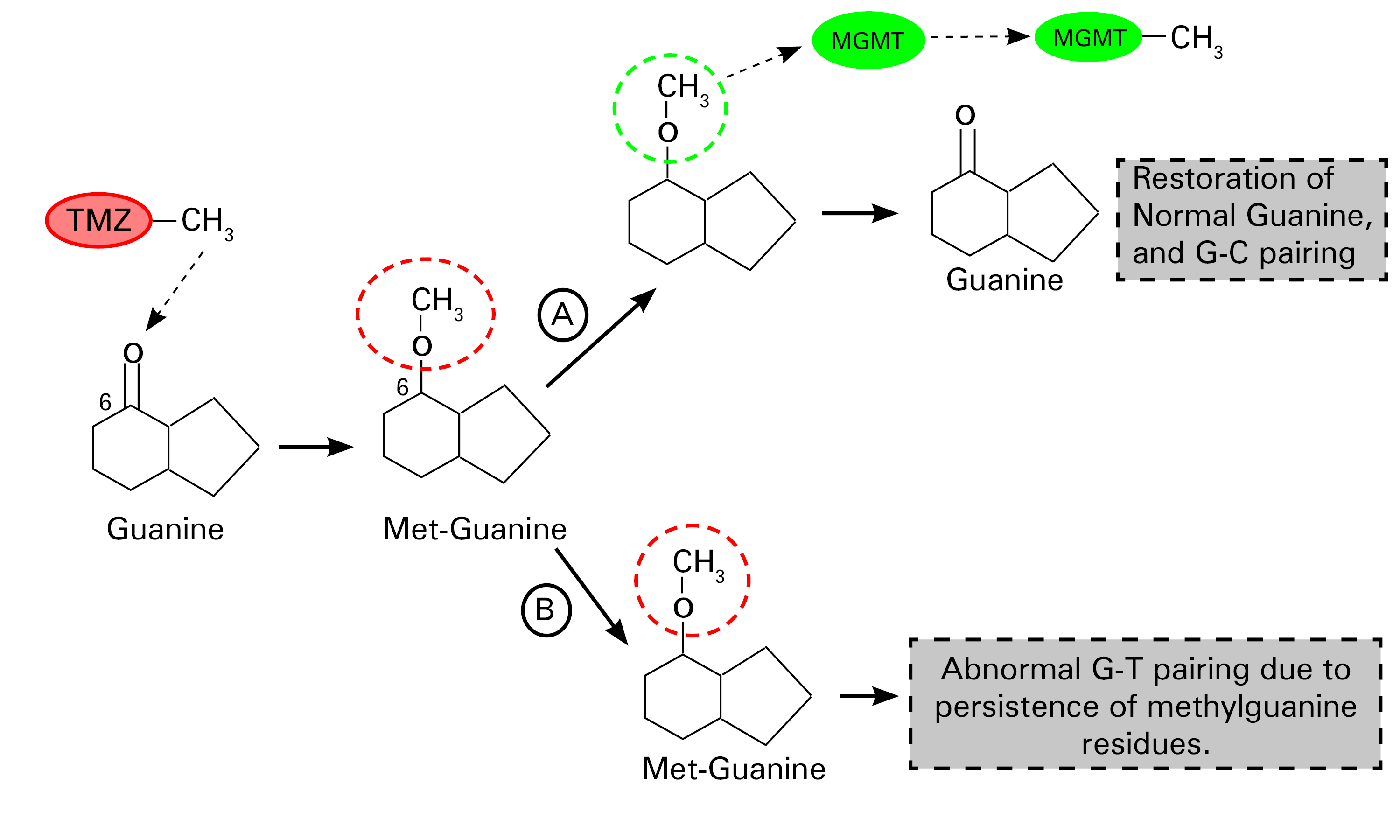
Schematic representation of the mechanism of action of temozolomide.
TMZ promotes the methylation of guanine(G) at the number 6 carbon position, a step that leads to the formation of methylguanine residues in DNA. There is a "suicide enzyme" called methylguanine-DNA methyltransferase (MGMT), whose primary function is to remove these abnormal methyl groups, thus restoring the integrity of guanine residues in DNA (Step A). This defective methylated guanine pairs with thymine (T) instead of cytosine (C) (by convention) during replication. Mismatch repair enzymes excise these mispaired guanine-thymine residues, although this is ultimately a futile exercise. Continuous cycles of erroneous pairing of G and T and T excisions lead to irreparable DNA breaks that promote cell death (step B) [12]. Redrawn and modified from Zhang et al. (2012) Temozolomide: Mechanisms of action, repair, and resistance. Curr Mol Pharmacol 5:102–114[12]
Evaluation of the methylation status of the MGMT promoter is a useful predictive biomarker[13] in patients with aggressive prolactinomas or carcinomas[14]. There is an inverse relationship between tumor levels of MGMT and the degree of responsiveness to temozolomide[14]. However, MGMT as a biomarker is a novel approach to predict the TMZ response, the therapeutic response after a minimum of 3 cycles of treatment performed better than the tumor levels of MGMT in a large cohort of patients with pituitary tumors (including prolactinomas)[15].
TMZ, a chemotherapeutic agent, is associated with expected short-term toxicity concerns, such as nausea, emesis, and fatigue[16]. Cytopenias (hematologic toxicity), which require a dose reduction or discontinuation of therapy, are infrequent[15,16]. Safety data after 5 to 8 years of exposure to TMZ are very reassuring, making it a valuable long-term salvage therapeutic option[17].
How does primary hypothyroidism contribute to hyperprolactinemia?
Undiagnosed primary hypothyroidism can present with significant hyperprolactinemia and pituitary gland enlargement[18,19]. Patients with primary hypothyroidism have an up-regulation of both TRH and TSH synthesis in the hypothalamus and pituitary gland, respectively[20]. TRH, a prolactin secretagogue, promotes hyperprolactinemia[21]. Anterior pituitary thyrotrope hyperplasia under the influence of TRH leads to the formation of a thyrotrope pseudotumor (diffuse enlargement of the pituitary gland). An inadvertent "stalk effect" due to impingement of the sellar mass on the pituitary stalk disrupts the dopaminergic tracts, leading to impaired tonic inhibition of lactotrophs by dopamine.
References
Kindly Let Us Know If This Was helpful? Thank You!


