The Intricacies of Insulin Synthesis, Storage, and Release
Insulin, the blood sugar regulator, is a 51 amino acid polypeptide born in the β-cells of the pancreas. The insulin story begins with a precursor molecule, proinsulin, which evolves from a larger insulin gene product, preproinsulin. The structure of proinsulin is intriguing; It includes an A-chain and a B-chain, interconnected by two disulfide bridges, and complemented by a 31 amino acid intermediate known as the connecting peptide or C-peptide. Within the A-chain portion, an extra disulfide bridge also appears.
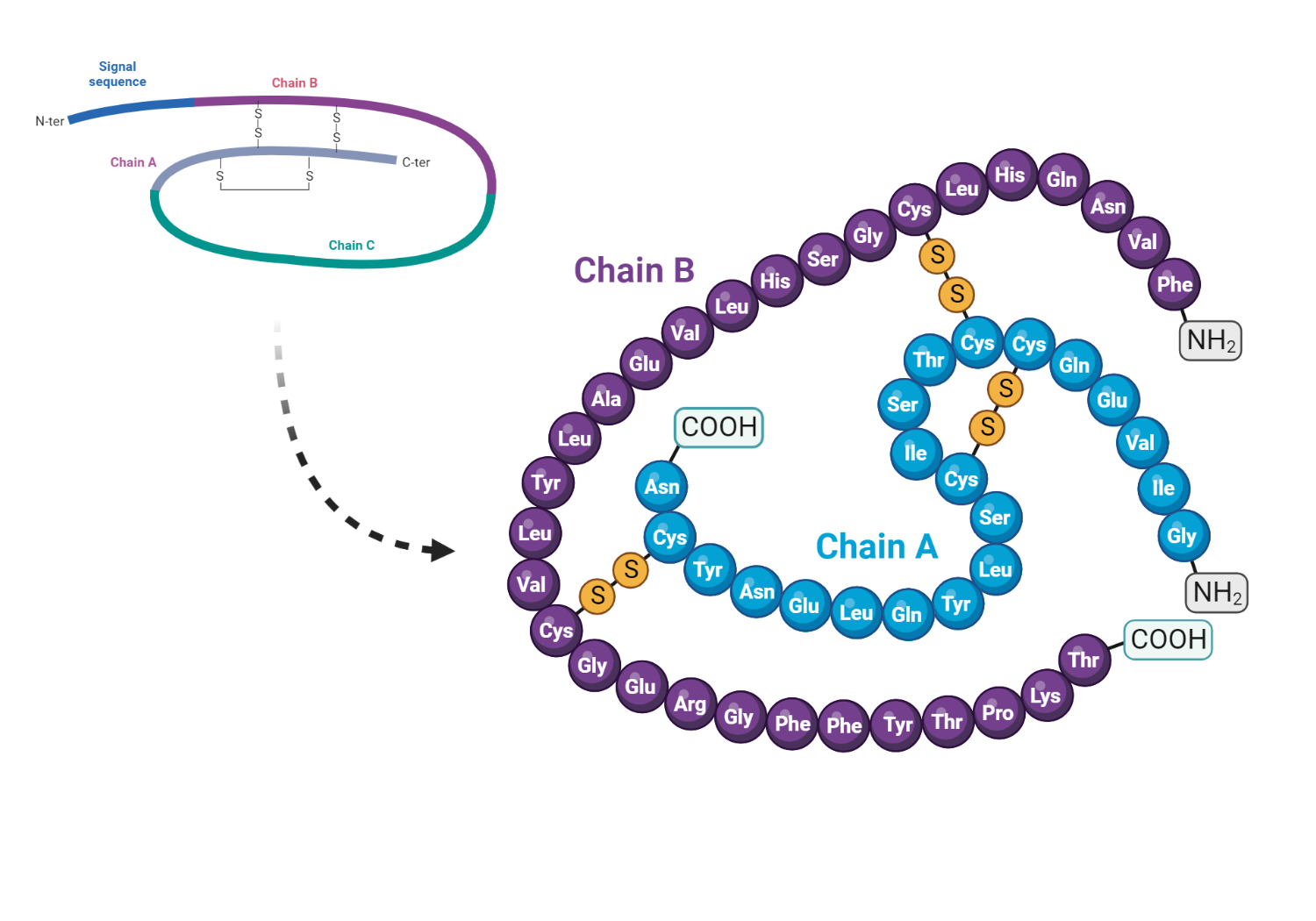
After its synthesis, proinsulin finds a home in cytoplasmic secretory granules located close to the cell membrane. Here, the C-peptide slowly cleaves away under the action of peptidase enzymes, allowing proinsulin to metamorphose into insulin and C-peptide molecules. Interestingly, insulin remains in the core of the granule, forming a polymeric complex along with zinc.
Insulin release is a coordinated dance. It is released in a calcium-dependent manner by exocytosis, along with equimolar amounts of inactive C-peptide and some proinsulin. Once in the portal circulation, insulin does not linger, it is rapidly metabolized by the liver and kidneys, boasting a plasma half-life of roughly 5-10 minutes. In clinical applications, the presence of C-peptide in the blood can be used to gauge β-cell activity in diabetic patients.
Regulation of insulin release is a nuanced process controlled by several stimulatory and inhibitory factors. A baseline level of secretion is consistently maintained, but several factors can influence the amount of insulin released. Among these, blood glucose concentration plays an important role. As one of the key physiological determinants of insulin secretion, the circulating glucose level directly stimulates both the synthesis and the release of insulin through a feedback mechanism.
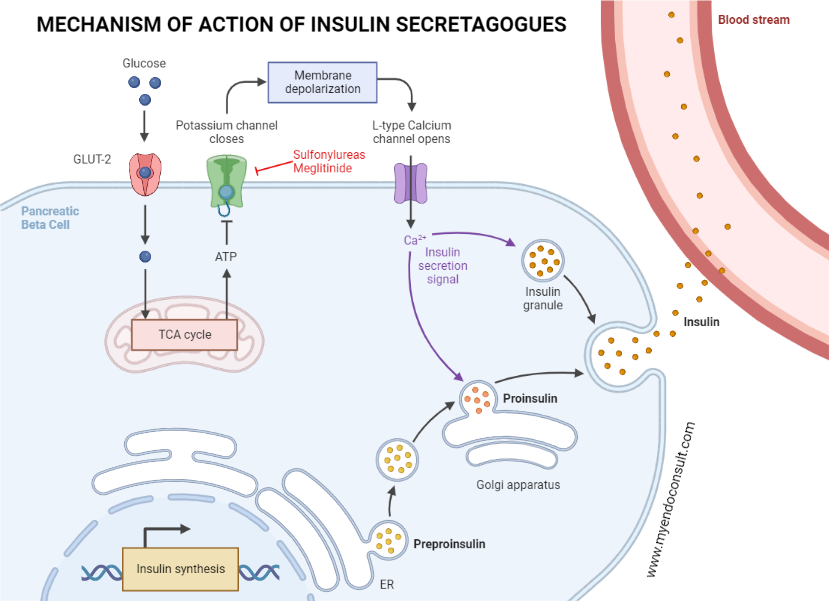
This stimulatory effect of glucose hinges on its initial facilitated transport to the β-cell via the GLUT2 carrier protein and subsequent metabolism to increase intracellular ATP levels. Higher ATP levels then stifle the activity of ATP-sensitive potassium channels (KATP) present in the β-cell membrane. This leads to cell depolarization, an influx of calcium, and, ultimately, to the release of insulin. Under normal ATP levels, these channels remain open, thus maintaining the β-cell in a hyperpolarized state.
Unraveling the Diverse Influencers of Insulin Release
Although insulin regulation is complex, other islet hormones, such as glucagon and somatostatin, subtly navigate this process. They indirectly influence insulin release by manipulating intracellular cAMP. Generally, an increase in cAMP, followed by activation of protein kinase A, encourages insulin exocytosis, and the opposite is true as well.
Gastrointestinal hormones also play a role in this intricate drama. Hormones such as gastrin, secretin, cholecystokinin, and especially gastric inhibitory peptide (GIP) emitted from mucosal endocrine K cells directly stimulate insulin release. These effects become particularly important when dealing with a sudden spike in blood glucose after a meal. Interestingly, an orally administered dose of glucose has been observed to provoke greater insulin release than its intravenously administered counterpart. This phenomenon underscores the significant role of gastrointestinal factors released during food consumption in increasing insulin secretion.
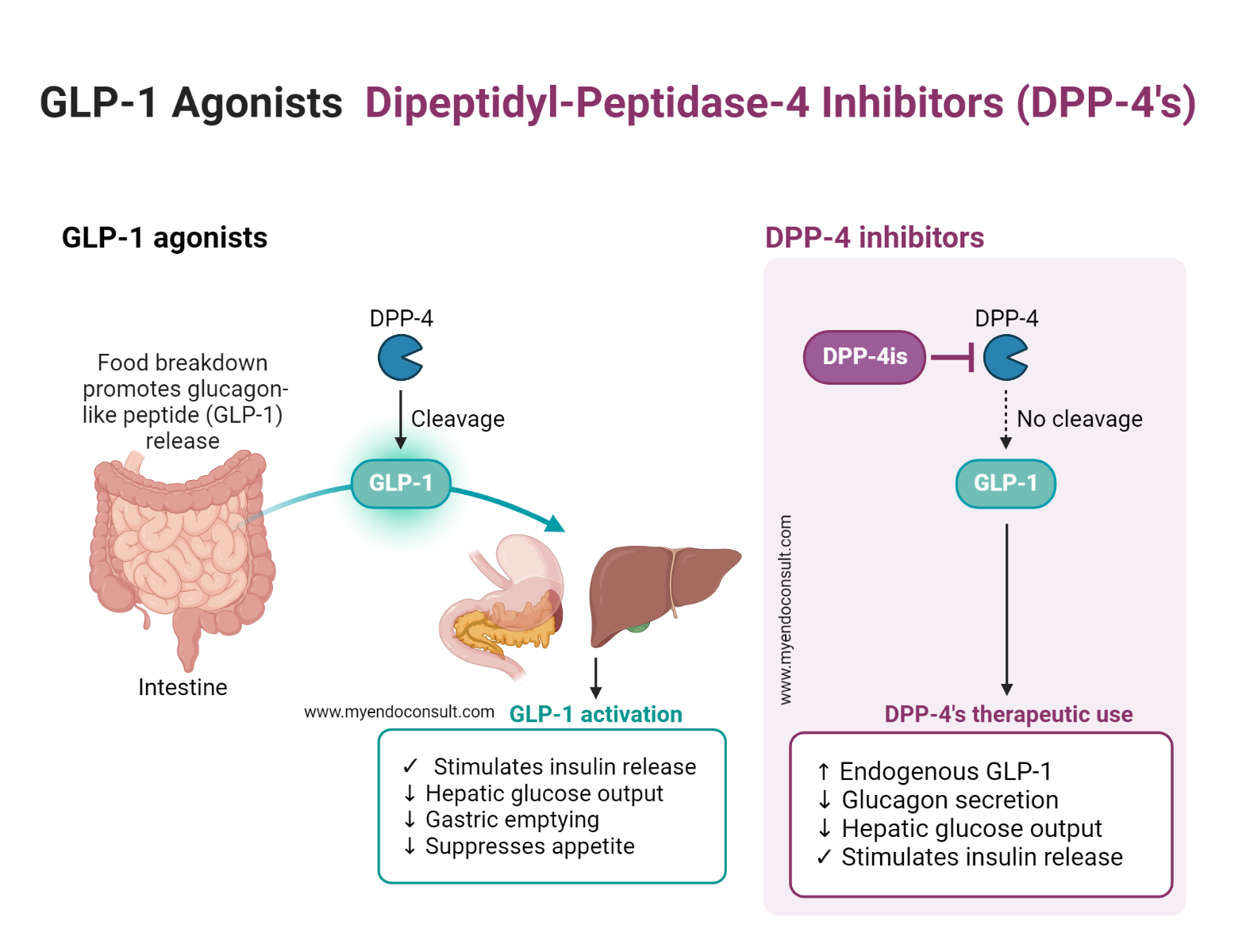
Deeper into the gastrointestinal angle, a truncated glucagon-like peptide (GLP-1 or incretin) is secreted by endocrine L cells of the lower intestine after meal or glucose ingestion. This peptide, derived from a preproglucagon precursor molecule, is a robust stimulator of insulin secretion from pancreatic β-cells. Its insulinotropic action is currently under investigation for potential therapeutic applications in the treatment of diabetes mellitus.
In addition, certain amino acids and fatty acids derived from protein digestion, such as arginine and leucine, are powerful stimulators of insulin release. They work synergistically with glucose to promote insulin secretion. Additionally, an increase in free fatty acid levels also encourages insulin release.
Other counterregulatory hormones, such as growth hormone (GH), glucocorticoids (cortisol), and thyroid hormone, indirectly stimulate insulin release by increasing the blood glucose level. In addition, the autonomic nervous system is actively involved in the process. Although parasympathetic nerve stimulation boosts insulin secretion, the dominant effect of sympathetic nerve stimulation is to impede release. This inhibition is due to the activation of α-adrenoceptors on the β-cell membrane, which results in a decrease in intracellular levels of cAMP. However, activation of β-adrenoceptors by adrenergic agonists produces the inverse effect.
Understanding the Chief Functions of Insulin
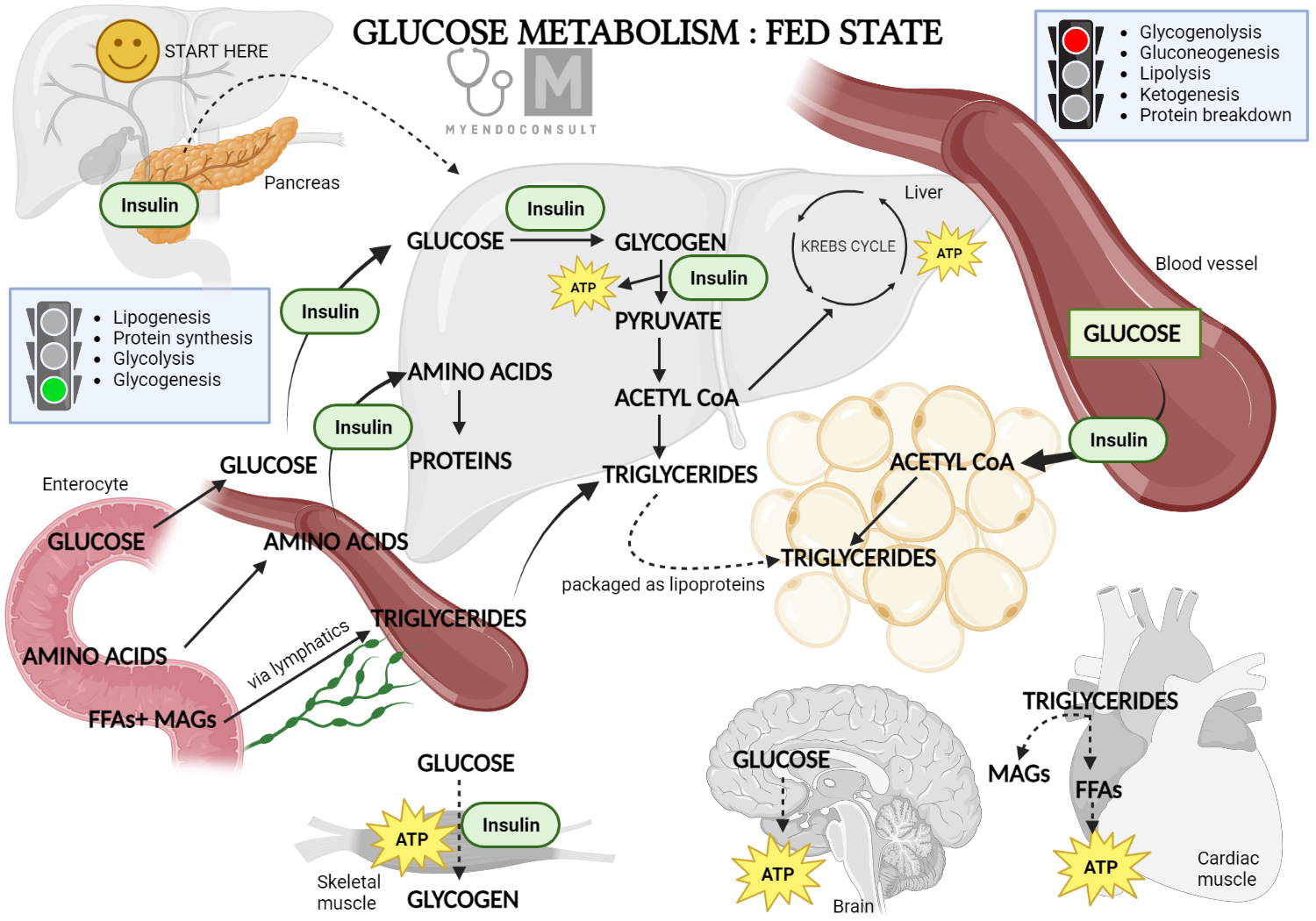
Insulin has the distinctive title of being the only hormone capable of directly decreasing blood glucose levels. In general, insulin is an anabolic hormone, endorsing the storage of chemical energy obtained from nourishment under the guise of glycogen, proteins, and lipids (triglycerides). At the same time, it inhibits the liberation or catabolism of stored nutrients. Consequently, the primary targets of insulin’s action are the liver, muscles, and adipose tissue, which are tailored for energy storage. However, not all tissues respond to insulin. Some, such as the kidney, brain, or red blood cells, demonstrate reduced sensitivity or are not responsive to this hormone.
Insulin’s principal impacts on target cells can be encapsulated into two main categories. First, insulin boosts the cellular transport of solutes, including glucose, amino acids, and fatty acids. Second, it modulates crucial intracellular metabolic pathways, amplifying the synthesis of glycogen, proteins, and fats. In addition, it enhances nucleic acid synthesis by directly stimulating the formation of DNA and RNA.
When we investigate carbohydrate metabolism, we find that insulin’s effects on glucose transport are quite remarkable. Under normal circumstances, plasma glucose is preserved within the range of 3.6 to 6.4 mmol / l (65 to 115 mg / dl). However, for certain tissues such as skeletal and cardiac muscle and adipose cells, glucose cannot infiltrate the cell membrane via simple or facilitated diffusion down its concentration gradient. As a result, intracellular glucose levels remain quite low. In the presence of insulin, glucose uptake by these tissues is rapidly increased due to the selective mobilization (and activation) of preformed glucose transporter molecules (GLUT4) from intracellular vesicles to the cell membrane. This process is postulated to be mediated by insulin-dependent phosphorylation of proteins that play a crucial role in vesicle translocation.
In summary, the role of insulin extends beyond its known responsibility for glucose regulation. By acting on key cellular processes, it orchestrates a complex symphony of metabolic pathways that ultimately ensure the body’s energy homeostasis.
Dissecting Insulin’s Pivotal Role in Carbohydrate Metabolism
Certain tissues, such as liver and red blood cells, and notably brain cells, rely solely on facilitated diffusion for glucose entry. These tissues employ specific glucose transporter proteins, which underscores the need to maintain an adequate blood glucose level to ensure their normal function. Interestingly, proximal tubule cells in the kidney and luminal epithelial cells in the small intestine have a unique insulin-independent, Na+-dependent glucose transporter (SGLT1).
Our knowledge of these transporter proteins has expanded with time, leading to the identification of at least five structurally related facilitative glucose transporter proteins, denoted as GLUT1-5. Each of these transporters displays a characteristic tissue distribution. However, among them, GLUT4 is unique. It is the only isoform that is expressed exclusively in skeletal muscle, cardiac muscle, and fat cells and is regulated by insulin.
Insulin’s multifaceted role extends to influencing the intracellular pathways involved in glucose and glycogen metabolism. Its diverse effects on carbohydrate metabolism can be illustrated by a few key points:
- Insulin promotes glycogen synthesis in skeletal muscle, liver, and adipose tissue. This is achieved by increasing the activity of glycogen synthase and decreasing the activity of glycogen phosphorylase.
- In the liver, insulin enhances glucose phosphorylation thanks to the increased activity of glucokinase. It also decreases glucose dephosphorylation by inhibiting glucose-6-phosphatase activity.
- Insulin increases glucose metabolism (glycolysis) while simultaneously attenuating liver gluconeogenesis. In the context of glycolysis, insulin increases the activity of key enzymes, such as 6-phosphofructokinase and pyruvate kinase, which facilitate the conversion of glucose to pyruvate and lactate. On the contrary, insulin inhibits enzymes required for gluconeogenesis, such as pyruvate carboxylase and phosphoenolpyruvate carboxykinase, curtailing the formation of glucose from amino acids, lactate, and glycerol.
In essence, insulin is a crucial orchestrator of metabolic pathways, exerting fine control over carbohydrate metabolism and ultimately contributing to maintaining the body’s metabolic equilibrium.
Insulin has a unique ability to reduce blood glucose levels in the body. It achieves this by limiting glucose release from liver glycogen stores and enhancing glucose uptake at the cellular level. Thus, it is clear to see why insulin is a major player in glucose and glycogen metabolism.
Moving onto protein metabolism, insulin showcases its proficiency in various ways. It stimulates the active transport of plasma amino acids into muscle cells, subsequently reducing the levels of amino acids in the blood. In addition, insulin directly stimulates muscle protein synthesis, displaying its anabolic abilities while also decreasing protein breakdown. The resulting decline in circulating amino acids plays a vital role in curtailing liver gluconeogenesis, another testament to insulin’s comprehensive influence.
Turning our attention to fat metabolism, we see further evidence for insulin’s critical role. Insulin promotes glucose uptake in adipose cells and promotes the synthesis, or lipogenesis, and storage of fatty acids as triglycerides in adipose and liver tissues. This action is supported by an increase in insulin-induced activity of lipoprotein lipase, a blood capillary enzyme that helps eliminate dietary fat from the plasma.
At the same time, insulin stops fat breakdown, or lipolysis, by inhibiting the activity of hormone-sensitive lipase in adipose cells. This dual action results in a decrease in circulating free fatty acids, a vital aspect that prevents the formation of plasma ketone bodies.
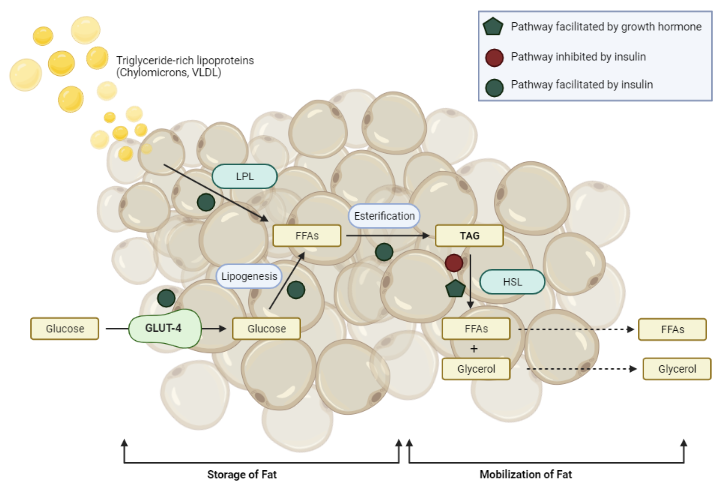
In summary, insulin’s role in the body is an impressive balancing act, managing carbohydrate, protein, and fat metabolism with exquisite precision. It is a vital hormone that underpins our body’s energy storage and use, orchestrating an intricate dance of metabolic processes.
How Insulin Works: Unveiling the Mystery Behind Insulin Action
Insulin, with its myriad effects on a variety of tissues, undoubtedly plays a central role in body metabolism. It accomplishes its diverse array of functions by interaction with specific insulin receptors located on the surface of target cells.
The insulin receptor is a large transmembrane glycoprotein consisting of two identical alpha-subunits and two identical beta-subunits connected by disulfide bridges. The alpha subunits, which bear high-affinity insulin binding sites, reside externally, while the beta subunits, housing an inherent tyrosine-specific protein kinase activity regulated by insulin binding, span the cell membrane. This kinase activity is vital for most of insulin’s metabolic actions. It is worth noting that some rare genetic disorders characterized by cellular insulin resistance are thought to stem from abnormalities in the structure and function of these insulin receptors.
To appreciate insulin’s actions, it is essential to understand the roles of protein kinase and phosphatase enzymes. Protein kinases transfer phosphate groups from ATP to amino acid residues on proteins. However, phosphatase enzymes hydrolytically remove phosphate groups, effectively reversing the regulatory effects of the kinases.
Once insulin binds to an alpha subunit, the internal beta-subunit domain of the adjacent alpha-beta dimer undergoes rapid phosphorylation, a process known as autophosphorylation. This, in turn, stimulates the inherent tyrosine kinase activity of the receptor.
A crucial intermediate cytoplasmic protein substrate for insulin receptor kinase is the insulin receptor substrate-1 (IRS-1), a peptide with multiple potential tyrosine phosphorylation sites within its molecule. The phosphorylated sites in IRS-1 bind with high affinity to specific cellular signaling proteins that eventually mediate the multifaceted insulin response.
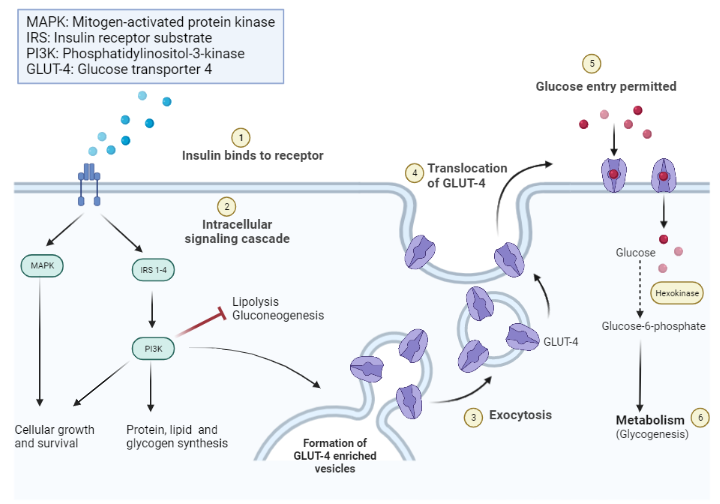
Although IRS-1 does not have an intrinsic catalytic function, it serves as a multisite ‘docking’ protein, interacting with various regulatory elements during insulin signal transmission. Some of the key enzymes and proteins that undergo phosphorylation or dephosphorylation in response to insulin are of significant interest to researchers.
Interestingly, the receptor for an insulin-like growth factor (IGF-1) shares many structural similarities with the insulin receptor and also uses the IRS-1 signaling pathway. This overlap provides further insight into the interconnected nature of our body’s regulatory systems.
Unraveling the Complexity of Insulin Action: From receptor binding to cell response
When insulin stimulates the insulin receptor for an extended period, the insulin-receptor complex undergoes internalization, a process known as endocytosis, which facilitates insulin degradation. This mechanism may serve as a termination point for the insulin signal. The reversal of insulin’s effects may also involve the action of various phosphatases.
Once the insulin-receptor complex is internalized, some of the receptors are degraded, but the majority are recycled back to the plasma membrane. Interestingly, the internalized insulin receptor retains its ability to function as a tyrosine kinase, suggesting that it may have additional functions in the signal transduction process.
Despite our current understanding of the insulin action pathway, there are still areas that remain somewhat ambiguous. For example, the precise mechanism by which insulin influences DNA and RNA synthesis is not entirely clear.
To better understand the cellular mechanism of insulin’s action, let us delve into how it interacts with its receptor. When insulin binds to one external alpha subunit of the insulin receptor, it causes a conformational change, leading to autophosphorylation of three tyrosine residues on the adjacent beta subunit. This triggers intrinsic tyrosine kinase activity.
Tyrosine phosphorylation of the main insulin receptor substrate, IRS-1, enables it to function as an intermediate signaling molecule. This leads to the activation or deactivation of key metabolic enzymes involved in mediating the insulin response. This process is a testament to the intricate orchestration that underpins the actions of insulin at the cellular level.
Clinical Applications
Variations of insulin have been synthesized by replacing or modifying one or more of its amino acids, leading to slight changes in its biological properties, such as its speed of absorption or duration of action. These are known as insulin analogs and are used to more accurately mimic the body’s natural pattern of insulin release.
Here are some examples of amino acid substitutions in insulin analogs:
- Insulin Lispro (Humalog): A rapid-acting insulin analog created by switching the places of proline and lysine in positions 28 and 29 of the insulin B chain.
- Insulin Aspart (Novolog): This rapid-acting insulin analog has an aspartic acid substituted for proline at position 28 of the B chain.
- Insulin Glulisine (Apidra): Another rapid-acting insulin analog, with an asparagine at position B3 replaced by a lysine and a lysine in position B29 replaced by glutamic acid.
- Insulin Glargine (Lantus): A long-acting insulin analog where asparagine at position A21 is replaced by glycine, and two arginines are added to the C-terminus of the B chain.
- Insulin Detemir (Levemir): In this long-acting insulin analog, a fatty acid chain is attached to lysine at position B29.
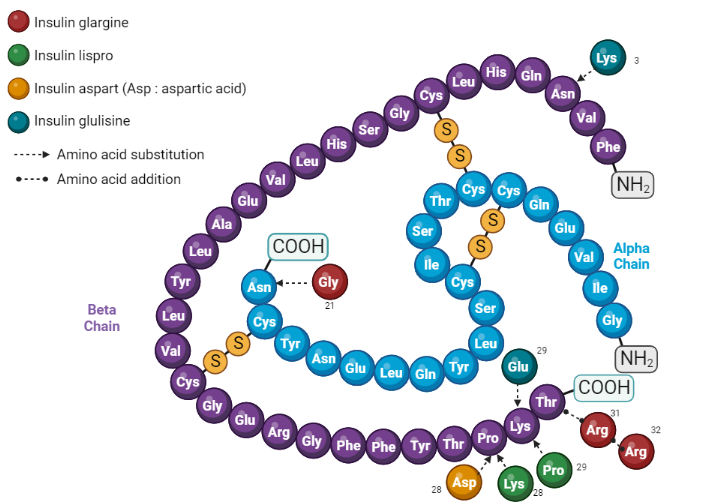
These insulin analogs have been engineered to optimize the pharmacokinetics of insulin for therapeutic use, providing options for faster onset or longer duration, depending on the needs of the patient. However, it’s important to note that any changes to insulin’s structure should be done under rigorous testing and regulatory oversight, as changes can potentially affect insulin’s ability to lower blood glucose or its potential to elicit an immune response.
Kindly Let Us Know If This Was helpful? Thank You!


