Basic Science Physiology of Insulin Action
Insulin's Composition
Insulin is a hormone composed of 51 amino acids, forming two chains—A and B—interlinked by a pair of disulfide bonds. These chains, containing 21 and 30 amino acids respectively, are connected by a sequence of amino acids named the connecting peptide or c-peptide. The gene responsible for human insulin creates an mRNA transcript, which is translated into a 110 amino acid polypeptide sequence called preproinsulin. Further modifications to preproinsulin happen in the rough endoplasmic reticulum, resulting in proinsulin. The Golgi apparatus then transports proinsulin where it's split into the insulin peptide and c-peptide. Pancreatic beta cells package these products along with amylin and other peptides into secretory granules.
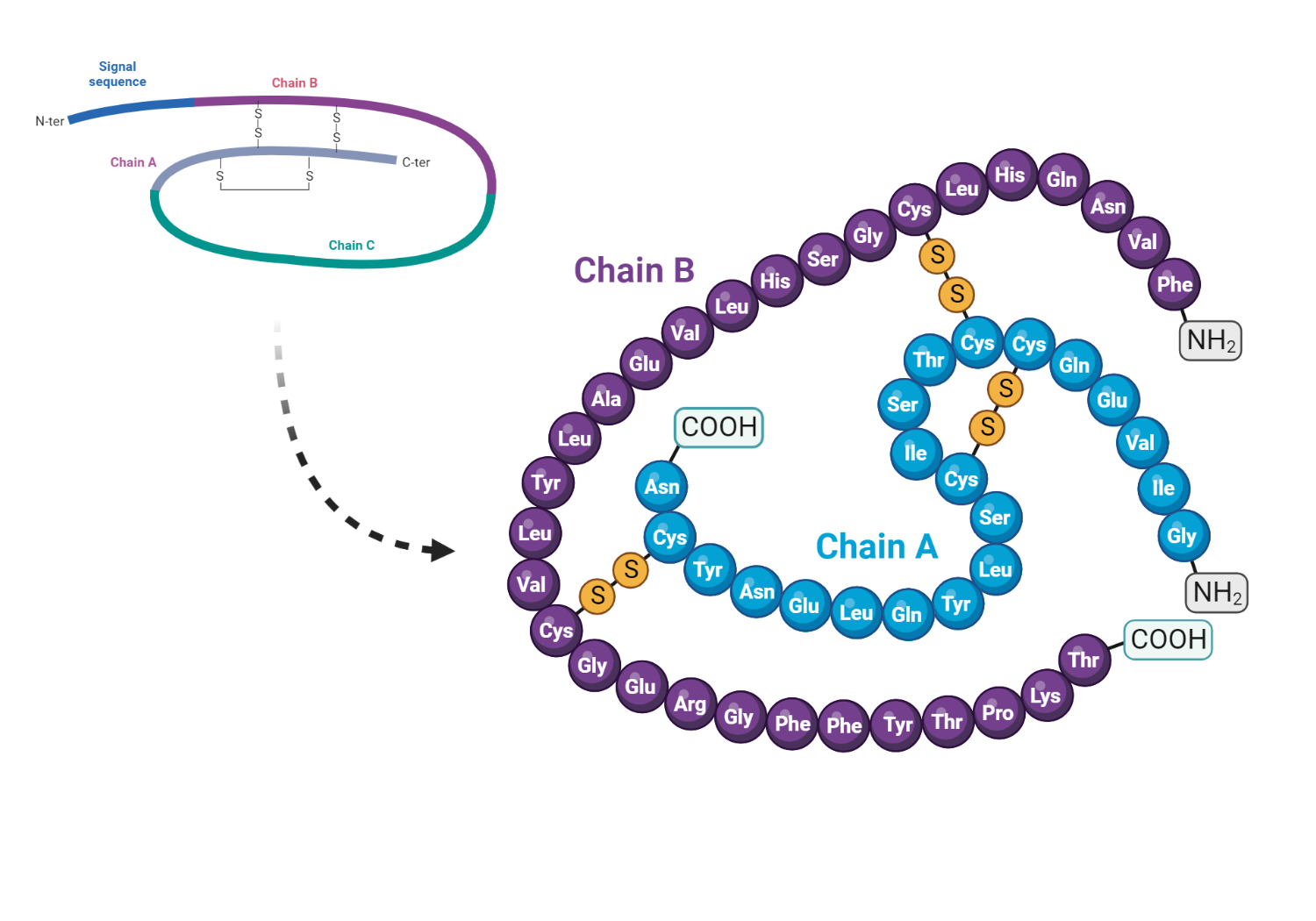
Structure of insulin showing the A and B chains with the interconnecting C peptide
Blood Glucose Regulation
The maintenance of glucose levels in the blood is primarily dependent on insulin, as well as other regulatory hormones. This system relies on the interplay of hormones, neural stimuli, cytokines, and other regulatory factors operating in various organs to keep plasma glucose under control. The pancreatic beta cell plays a critical role in this complex system.
In the fasting state, decreased insulin levels lead to the burning of fatty acids in fat tissues, making them a key energy source. The liver uses these fatty acids for producing glucose during prolonged fasting. However, the brain requires glucose, necessitating alternative energy sources during fasting. The liver provides glucose during extended fasting. The pancreas releases glucagon during fasting, promoting gluconeogenesis and glycogenolysis in the liver, thus maintaining glucose levels in the blood.
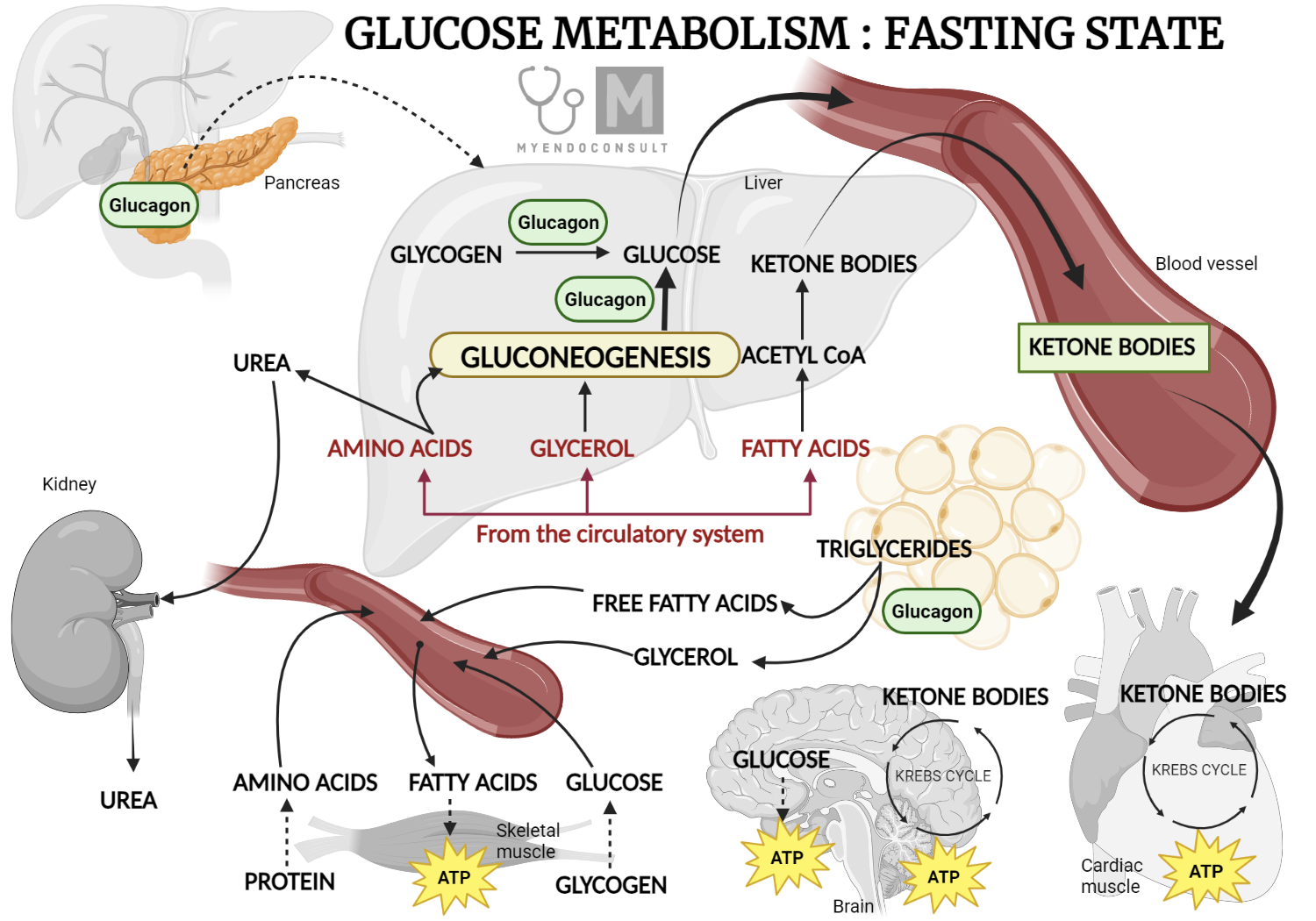
Glucose Metabolism Fasting State
In the post-meal state, the pancreatic beta cell senses glucose, triggering the release of insulin. Insulin then performs several metabolic functions, such as reducing hepatic glucose production, promoting glucose absorption in peripheral tissues, and decreasing lipolysis in fat tissues. The impacts of insulin on various tissues, including skeletal muscle, adipose tissue, and liver, are explored below.
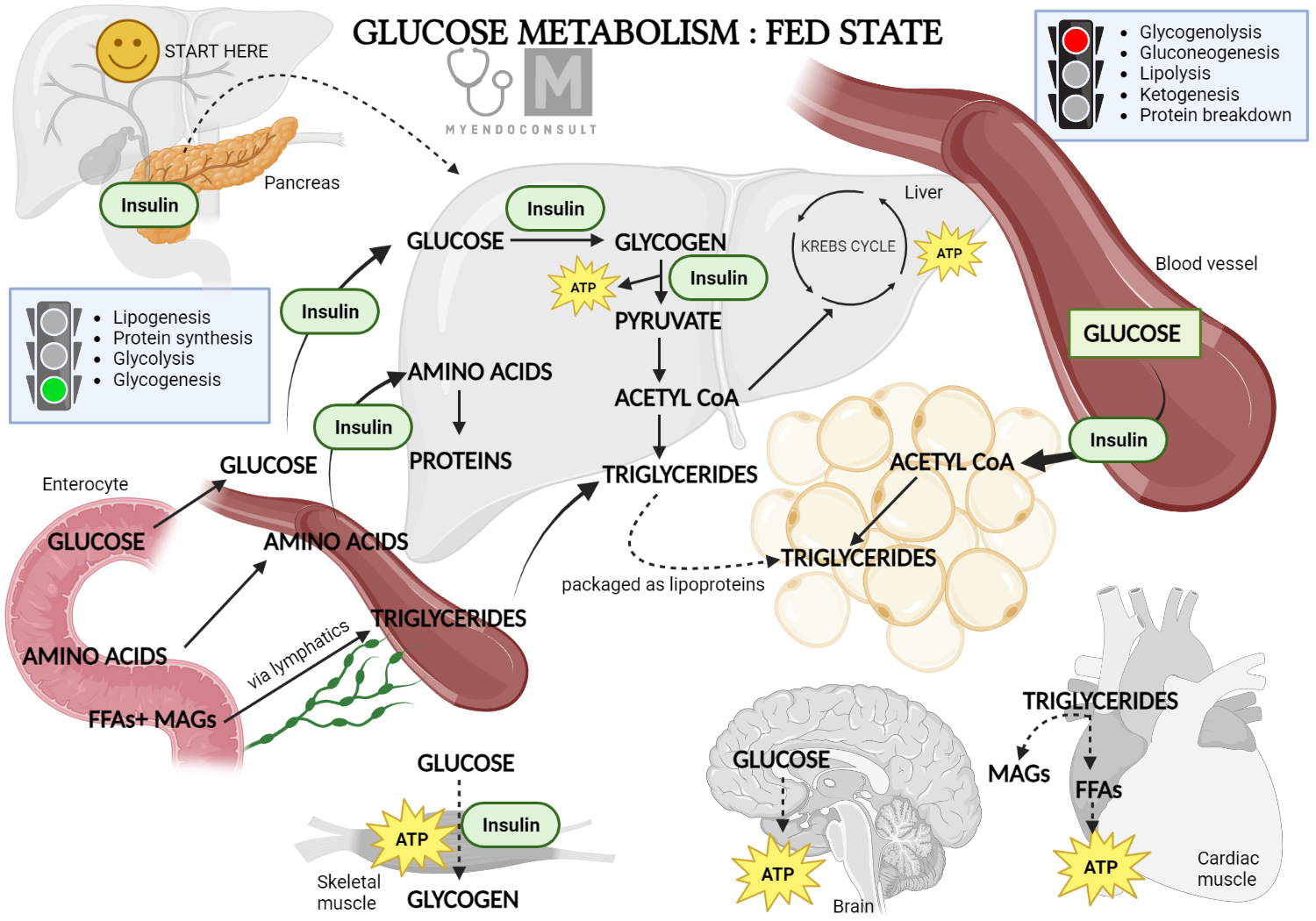
Glucose Metabolism Fed State
Insulin's Impact on Different Tissues
Skeletal muscle primarily utilizes glucose and free fatty acids for energy during the post-meal and fasting states, respectively. Skeletal muscle is the main site of glucose absorption after a meal, with insulin being primarily responsible for this function. An increase in blood glucose following a meal is detected by pancreatic beta cells, which then release insulin. Insulin then initiates a signaling process that results in the transport of the glucose transporter 4 (GLUT 4) to the plasma membrane of the skeletal muscle. GLUT-4 then facilitates glucose absorption by the muscle.
After entering skeletal muscle cells, glucose is converted by the hexokinase enzyme into glucose-6-phosphate, which can either be stored as glycogen or used in the glycolytic pathway. A significant portion of glucose in this pathway is oxidized to produce energy, with a small amount being converted into lactate.
During fasting, decreased insulin levels limit the hormone's anti-lipolytic effects in white fat tissue. This leads to increased lipolysis in the tissue and the generation of fatty acids, which serve as the main fuel source for skeletal muscle.
The liver acts as the main source of glucose production during fasting, ensuring that cells reliant on glucose, such as neurons, red blood cells, and renal medulla cells, function optimally. This is achieved by increasing glycogenolysis, gluconeogenesis, and glycogen synthesis in the period following absorption. This system relies on the interaction of hormones, substrates, and allosteric factors, the details of which are beyond the scope of this discussion.
During the period following a meal, insulin facilitates several actions that diminish the liver's glucose production.
- One of the mechanisms involves insulin's antilipolytic effect, which prevents the conversion of triglycerides into free fatty acids in white fat tissue. As a result, the amount of free fatty acids, a primary ingredient for the liver's glucose production, is lessened, leading to a decrease in glucose production in the liver.
- Another mechanism revolves around glucagon, a hormone that increases glucose production in the liver. After a meal, higher levels of insulin in the blood inhibit the pancreas's alpha cells from releasing glucagon. This action, in turn, results in a reduced output of glucose from the liver.
The Insulin Receptor
The insulin receptor (IR) consists of two distinct parts: an external component made up of two alpha subunits, and a part that crosses the cell membrane, which includes two beta subunits. In typical bodily functions, the alpha subunits suppress the inherent tyrosine kinase activity of the beta subunits.
Upon binding with insulin, the alpha subunit of the IR experiences a reduction in its capacity to restrain the beta subunit. As a result, when insulin binds to the IR, it encourages the phosphorylation of internal proteins necessary for various subsequent signaling processes in different tissues. The activation of the insulin receptor leads to various effects within the cell, including the enhanced synthesis of glycogen, protein, and lipids. Meanwhile, lipolysis and gluconeogenesis processes are curtailed. Additionally, insulin encourages the mobilization of glucose transporter channels on peripheral tissues, such as skeletal muscle.
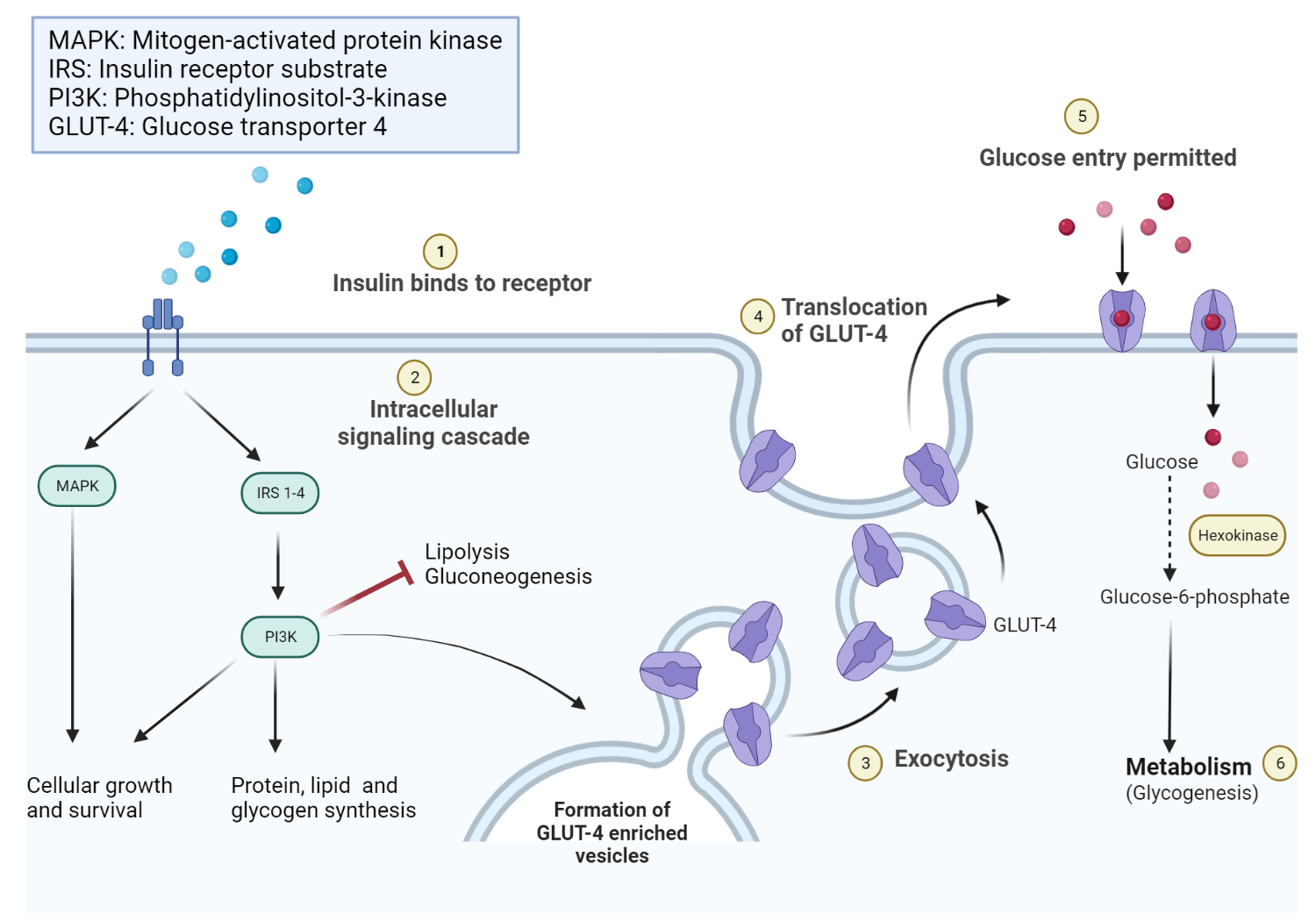
Mechanism of action of insulin
Mechanism of Action of Insulin
A significant part of our comprehension of chronic high blood sugar in individuals with type 2 diabetes is insulin resistance. This condition is defined by the inability of regular insulin levels in the blood to lower glucose levels during the post-meal state. Consequently, enhanced liver glucose production, increased lipolysis, and compromised peripheral glucose uptake contribute to high blood sugar.
Insulin resistance in type 2 diabetes manifests differently in various tissues:
- In skeletal muscle, there is a problem with the transportation of the glucose transporter 4 (GLUT4) to the cell membrane of muscle cells and a defect in the tyrosine phosphorylation of several insulin receptor substrates. This leads to a decrease in glucose uptake by the skeletal muscle.
- In adipose tissue, there is a diminished response to the anti-lipolytic action of insulin due to an unknown mechanism. Release of adipokines associated with insulin resistance contributes to increased lipolysis.
- In the liver, adipokines and free fatty acids originating from adipose tissue disrupt insulin signaling. This leads to a lack of control over liver glucose output, resulting in constant high blood sugar.
Therapeutic Insights for Insulin Use
Insulin therapy can either complement or be the primary treatment for managing type 2 diabetes. However, insulin treatment is essential to prevent dangerous ketoacidosis in patients with either structural or functional issues with the pancreas (such as type 1 diabetes).
Even though backed by clinical evidence, the recommendations for insulin use in type 2 diabetes differ substantially among various clinical practice guidelines. It is commonly agreed upon, though, that insulin is initiated in patients likely to experience beta cell failure and worsening high blood sugar, even when taking optimized doses of non-insulin therapies.
Patients with type 1 diabetes need both basal and mealtime insulin if they are on multiple daily insulin injections. The standard initial total daily insulin dose is 0.5 units per kg of body weight per day. This dose is divided into basal (40-60%) and bolus (40-60%) insulin. Patients with good carbohydrate counting abilities may use a carbohydrate ratio to estimate the amount of bolus insulin.
Kindly Let Us Know If This Was helpful? Thank You!


