Delving into the Parathyroid Glands
The human body is home to numerous specialized organs, each performing distinct roles. Among these are the parathyroid glands, four small, ovoid structures positioned in the posterior region of the thyroid gland, encapsulated within the thyroid capsule. Despite their size, comparable to that of a pea, these glands play a vital role in the regulation of calcium and phosphate metabolism.
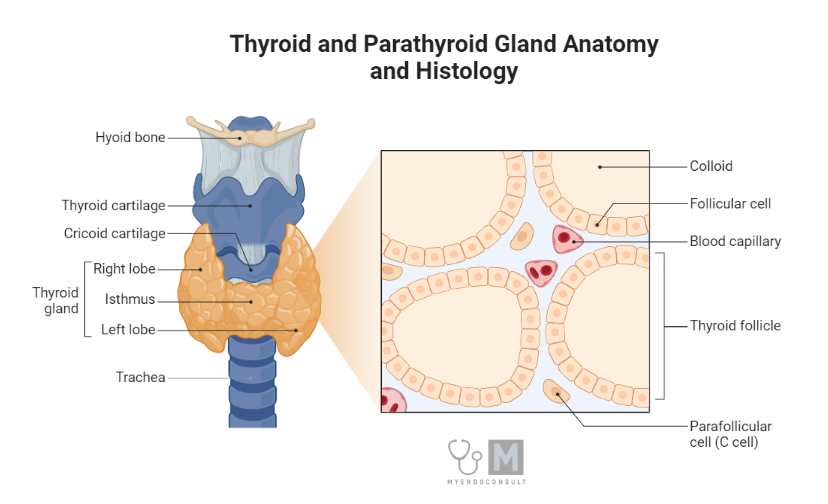
The parathyroid glands are composed of two types of epithelial cells—chief cells and oxyphil cells. The more abundant chief cells are characterized by a clear cytoplasm containing dense granules, from which they secrete parathyroid hormone (PTH). The larger oxyphil cells, though less granular, currently don’t have an established hormonal function.
PTH, an 84 amino acid linear peptide, is synthesized from larger precursor polypeptides—prepro-PTH and pro-PTH. Upon its release, PTH is primarily cleaved by liver and kidney cells into an active N-terminal fragment and an inactive C-terminal fragment. This N-terminal fragment contains the first 34 amino acids, with residues 1–27 being essential for its functionality. Intriguingly, human PTH shares structural similarity with porcine and bovine PTH.
PTH’s secretion process differs from other endocrine hormones; it isn’t regulated by the anterior pituitary gland. Instead, it is guided by the circulating blood level of ionized calcium. Lower levels of plasma calcium stimulate PTH release, while elevated levels suppress its release—a mechanism of negative feedback. This response appears to involve a G-protein coupled cell-surface receptor for calcium. Moreover, plasma magnesium exerts a comparable, albeit weaker, effect on PTH secretion.
The physiological relevance of this effect, however, remains unclear. Plasma phosphate levels do not directly affect PTH secretion. However, it’s worth noting that 1,25-dihydroxyvitamin D3, an active vitamin D metabolite, can potentially reduce PTH production by the parathyroid glands.
The principal function of PTH is to elevate the plasma ionized calcium concentration, hence serving as a calcium-raising factor. PTH accomplishes this by influencing bone and renal tubular reabsorption of calcium and increasing intestinal absorption. Concurrently, PTH reduces plasma phosphate levels, further emphasizing its critical role in calcium and phosphate homeostasis.
Understanding the Intricate Actions of Parathyroid Hormone
Parathyroid hormone (PTH), a critical regulator of calcium and phosphate metabolism, executes its multifaceted functions primarily in the kidneys, bones, and gastrointestinal tract. Despite the complexity of these actions, the driving force behind them is maintaining the balance of plasma calcium and phosphate concentrations.
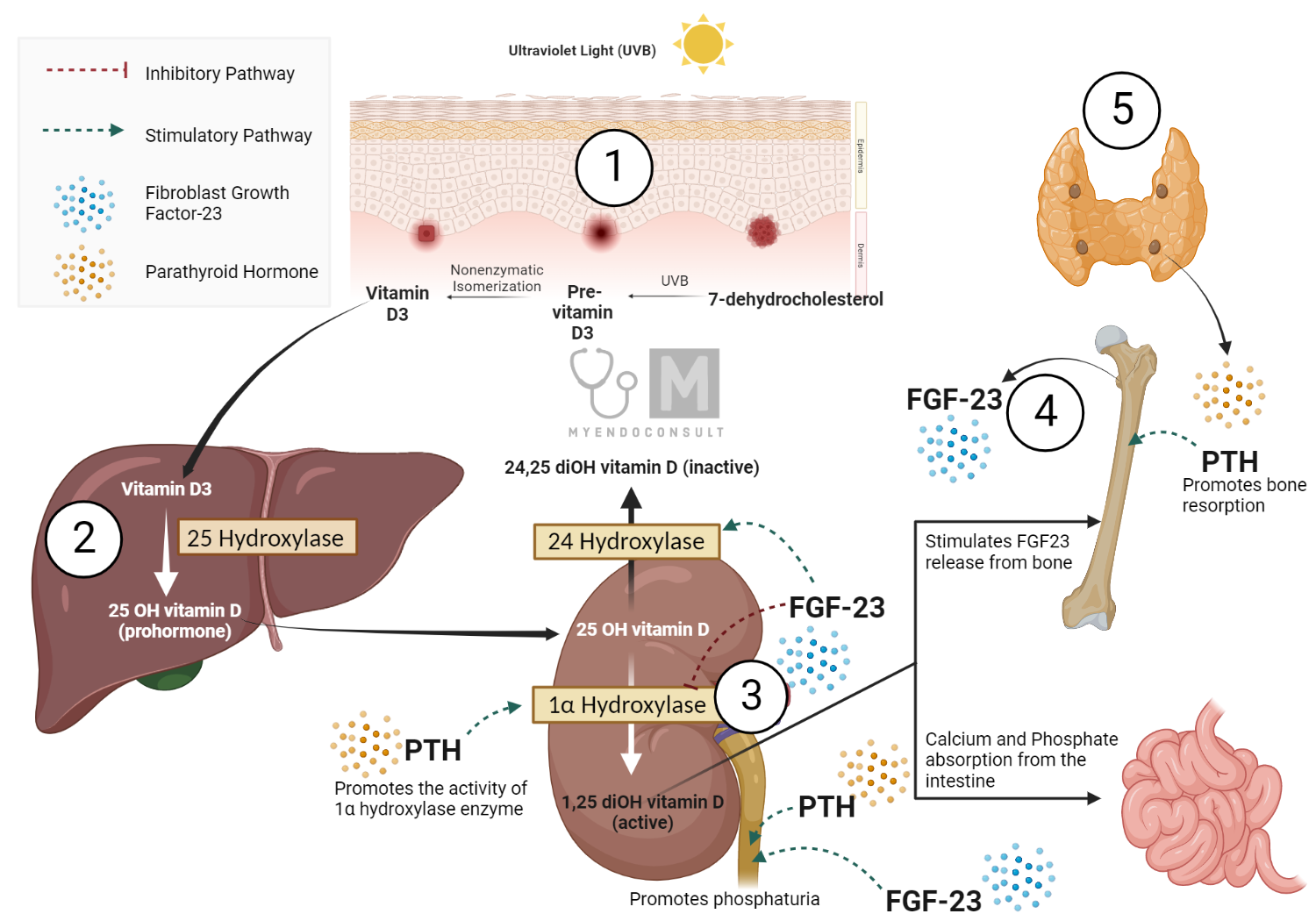
In the kidneys, PTH initiates a prompt increase in distal tubular reabsorption of calcium (and magnesium) from the glomerular filtrate. This hormone also amplifies the urinary excretion of phosphate by inhibiting phosphate reabsorption in the proximal tubule, a process mediated through a sodium/phosphate-cotransport mechanism. Consequently, PTH enhances plasma calcium concentration while diminishing plasma phosphate levels. Furthermore, PTH encourages the creation of the active vitamin D metabolite 1α, 25-dihydroxycholecalciferol within the kidney tubular cells, facilitating the release of calcium from the bone and augmenting the absorption of calcium from the small intestine and kidney. This synthesis is mediated through the activation of the renal mitochondrial enzyme 25-hydroxyvitamin D-1α-hydroxylase.
On the bone front, PTH exhibits dual functionality. Initially, it triggers the swift efflux of calcium from the labile calcium pool across the osteocyte-osteoblast bone membrane into the extracellular fluid. The vitamin D metabolite 1,25-dihydroxycholecalciferol facilitates this process. Additionally, PTH instigates the osteoclast cells to release calcium and phosphate via a slower bone dissolution or resorption process. This action also boosts the number of osteoclasts involved. Over time, osteoblast cells are encouraged to synthesize new bone matrix, underscoring PTH’s involvement in bone remodeling.
In the context of the gastrointestinal tract, PTH indirectly stimulates the absorption of calcium and phosphate. This effect, requiring approximately 24 hours, is mediated by the vitamin D metabolite 1,25-dihydroxycholecalciferol and, therefore, is absent in vitamin D-deficient states. PTH facilitates the activity of the enzyme 1α-hydroxylase, crucial for the production of 1,25-dihydroxycholecalciferol in the kidneys. Thus, PTH forms an interconnected network with other key players in the regulation of calcium and phosphate homeostasis.
The Intricacies of Bone Remodeling: The Role of Parathyroid Hormone
Understanding bone remodeling, a process critical for preserving normal bone strength and calcium homeostasis, requires a deep dive into the two central cell types involved – osteoblasts and osteoclasts. Both of these play a pivotal role in maintaining bone structure, but their functions are orchestrated by the parathyroid hormone (PTH).
Osteoblasts are specialized, fibroblast-like cells that originate from marrow osteoprogenitor cells and are entrusted with new bone formation. These cells secrete type I collagen and glycoproteins to form osteoid in the extracellular space, which then undergoes calcification, creating an organic matrix that initially contains calcium phosphate and, later, hydroxyapatite crystals. Osteoblasts can transform into interior osteocytes if they become entirely encompassed by mineralized bone. These interior osteocytes maintain cytoplasmic contact with each other and with active osteoblasts through gap junctions, forming a ‘bone membrane’. The enzyme alkaline phosphatase, considered instrumental for normal bone mineralization, is present in osteoblast cells and can be measured in the plasma as an indicator of osteoblastic activity.
On the flip side, osteoclasts are larger, multinucleated cells originating from hemopoietic lineage. These cells line the bone-forming surface of bone tissue and are adept at digesting previously formed bone matrix, thus releasing calcium and phosphate into the extracellular bone fluid – a process termed bone resorption. Bone absorption transpires beneath the ruffled border area of the osteoclast, which dispenses hydrolytic enzymes as well as protons. The process of bone resorption is slow and continual, typically lasting between 2-4 weeks, followed by new matrix deposition and mineralization that may span 3-7 months. This cyclical process is termed as bone remodeling and is critical for maintaining bone strength, repairing microfractures, and preserving calcium homeostasis. The sites on the bone surface where remodeling occurs are termed basic multicellular units (BMUs).
It is widely suggested that PTH directly influences only osteoblast cells, and its effects on osteoclast bone resorption are indirectly mediated. This indirect pathway could involve the release of cytokines such as interleukins-1 and 6, and granulocyte macrophage-colony stimulating factor, which play a part in the activation and differentiation of osteoclasts from precursor cells.
The Multifaceted Manifestations of Hyperparathyroidism
Parathyroid carcinoma, though rare, can result in hyperparathyroidism. Additionally, this condition may also coincide with a broader inherited endocrine syndrome known as multiple endocrine neoplasia type 1 (MEN-1 disease) or multiple endocrine adenomatosis. This syndrome affects numerous endocrine glands, including but not limited to the pancreas, pituitary, adrenal, and thyroid glands.
Secondary overactivity and hyperplasia of all four parathyroid glands can arise as a compensatory response to prolonged hypocalcemia. This can occur due to intestinal calcium/vitamin D malabsorption or deficiency, or as a result of chronic renal failure. In these cases, Parathyroid Hormone (PTH) levels persist at elevated levels until the cause of the hypocalcemia is rectified.
Mild hyperparathyroidism may not present with many clinical signs initially. However, a chronic excess of PTH can induce symptoms that directly arise from elevated blood calcium levels. Recognizing these varied manifestations is critical to ensuring timely diagnosis and management of these conditions. As our understanding of these complex endocrine interplays deepens, so too does our capacity to deliver effective interventions.
Hyperparathyroidism: Symptoms, Treatment, and Future Directions
Hyperparathyroidism, a condition characterized by excessive parathyroid hormone (PTH) production, presents with a constellation of symptoms that are often multifaceted:
- General systemic symptoms include tiredness, lethargy, excessive thirst, and psychiatric disorders like depression and paranoia. Additionally, cardiac irregularities, anemia, and hypertension may also be present.
- Renal calculi, composed of insoluble calcium phosphate or oxalate, often occur due to increased urinary excretion of calcium and phosphate. This condition could cause renal colic and the presence of blood in urine, eventually leading to renal failure. Gallstones can also occur.
- Gastrointestinal complaints like abdominal pain, constipation, dyspepsia, as well as peptic and duodenal ulceration, may also be present.
- Excessive PTH can cause generalized loss of calcium from bones, leading to bone lesions. In severe cases, osteitis fibrosa with bone pain and increased incidence of fractures may occur.
This disease is commonly summarized as a condition of ‘bones, stones, and abdominal groans’.
The principal treatment of hyperparathyroidism involves surgical removal of the overactive parathyroid tissue, although locating some tumors can prove challenging. Parathyroid carcinomas can also be managed via local excision. However, postoperative persistence of hyper parathyroid symptoms or the development of permanent hypoparathyroidism is common.
Severe hypercalcemia, known as a hypercalcemic crisis, requires immediate management via oral or intravenous rehydration fluids, diuretics to increase urinary calcium excretion, and possibly salmon calcitonin or disodium pamidronate to inhibit bone resorption.
Hypoparathyroidism: Pathophysiology, Symptoms and Therapeutic Approaches
Hypoparathyroidism, characterized by a parathyroid hormone (PTH) deficiency, results in hypocalcemia and hyperphosphatemia due to increased renal tubular phosphate reabsorption. This disorder is most commonly associated with inadvertent damage to the parathyroid glands during thyroid surgery. However, a rare form of primary hypoparathyroidism can also emerge from autoimmune diseases, often coexisting with other organ-specific endocrine autoimmune disorders such as adrenal insufficiency, hypothyroidism, or type I diabetes. Such patients may exhibit serum antibodies against parathyroid tissue.
An unusual hereditary condition termed pseudohypoparathyroidism displays normal parathyroid function, yet the primary target tissues—bone and kidney—exhibit resistance to PTH actions. This resistance has been associated with a deficiency in the α-subunit structure of the stimulatory Gs-protein complex involved in PTH receptor transduction. Clinical features of this condition range from mild to severe hypocalcemia and include a rounded face, short stature, obesity, and short fingers. Some patients may also present with cognitive impairment.
A sustained low plasma calcium level can elicit:
- Nerve and neuromuscular tissue hyperexcitability, leading to paraesthesia in the face or fingers, muscle cramps, spasms, hypocalcemic tetany, and even seizures or convulsions. Electrocardiogram recordings may reveal a prolonged QT interval.
- Chvostek’s sign (facial muscle twitching in response to light tapping over the facial nerve) and Trousseau’s sign (tetanic spasm of the wrist and fingers following over-inflation of a sphygmomanometer cuff placed over the upper arm for over three minutes).
- In chronic cases, dental abnormalities, dry scaly skin and hair, brittle nails, and cataracts may also manifest.
Depending on the severity of hypocalcemia, treatment requires vitamin D supplements, such as calciferol tablets or an oral solution of the vitamin D derivative dihydrotachysterol, to augment calcium absorption and utilization. Concurrent serum and urinary calcium measurements are essential to prevent overtreatment and consequent hypercalcemia. In acute cases of hypocalcemic tetany, a rapid elevation in blood calcium can be achieved via an intravenous infusion of 10% calcium gluconate solution. As of now, PTH is not available for replacement therapy.
Kindly Let Us Know If This Was helpful? Thank You!


