Blood plasma is a pale, straw-colored fluid of water, proteins, hormones, and nutrients. In other words, blood plasma is blood without blood cells, of which 92% of plasma is water, 7% belongs to plasma proteins, and >1 % are regulatory proteins and other solutes.
Plasma proteins are carriers of many functions of the blood. This article aims to help you understand its structure and functions in physiological and pathological conditions.
Overview of plasma proteins
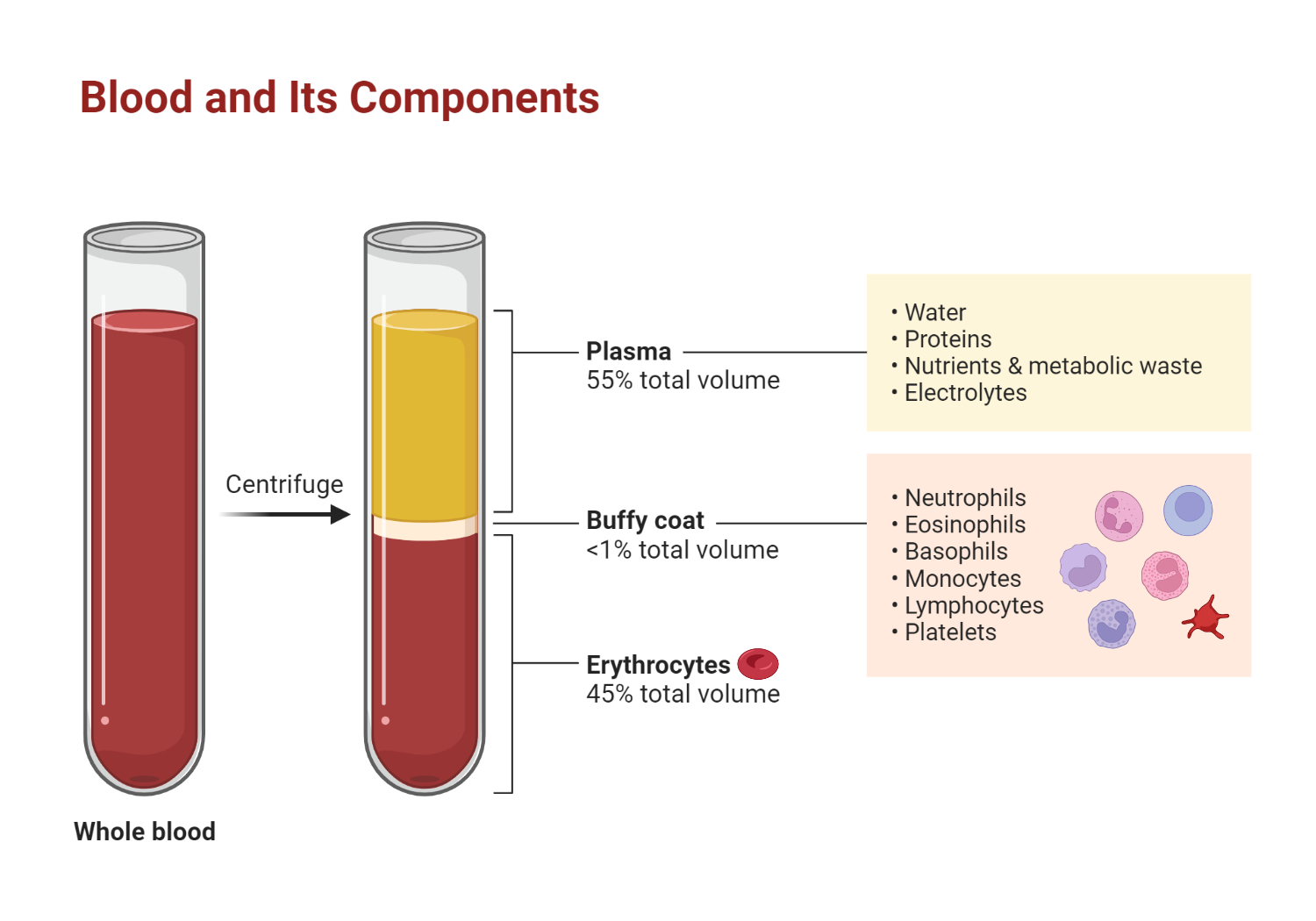
Figure 1.0 Components of blood
Blood plasma is the fluid in which the formed elements are suspended. It floats at the top of the tube, separated from the densest elements, erythrocytes, which are separated by a buffy coat of leukocytes and platelets. Hematocrit is the percentage of the total sample that consists of erythrocytes.
Although a small percentage (7%) belongs to plasma proteins, they perform many blood functions: the coagulation process, regulation of acid-base balance, defense against pathogens, transport of nutrients and hormones, excretion of waste products, regulation of osmotic pressure, control of erythrocyte sedimentation rate, etc.
What are proteins? They are essential molecules that consist of amino acid residues joined by peptide bonds.
Plasma proteins can be separated by one method known as electrophoresis, where they migrate as individual fractions based on their size and electrical charge. Three fractions of plasma proteins: albumins, globulins, and fibrinogen, are visible on an electrophoresis interface. Next, we review these individual components of plasma proteins, including their structure and biological function.
Albumin is the most abundant of plasma proteins and typically accounts for approximately 54% of the total plasma protein content. The liver produces it, and its role serves as a transport vehicle for fatty acids and hydrophobic steroid hormones. Moreover, albumin is the most significant contributor to the osmotic pressure of blood: holding the water inside the blood vessels helps maintain blood volume and blood pressure.
Next, we have the globulin fraction, a heterogeneous group that contains alpha, beta, and gamma globulins. Alpha and beta globulins transport iron, lipids, and fat-soluble vitamins such as K, A, D, and E. Gamma globulins are the central part of humoral immunity and are known as antibodies or immunoglobulins. Unlike alpha and beta globulins, which are produced in the liver, immunoglobulins are produced by transformed B cells, known as plasma cells.
Fibrinogen is the least abundant plasma protein, taking 7% of the total plasma protein volume. Fibrinogen, a liver product, is the first factor in the coagulation cascade process.
Plasma proteins involved in hormone transport include corticosteroid-binding globulin, thyroxine-binding globulin, insulin-like growth factor-binding protein 3, sex hormone-binding globulin, and alfa fetoprotein, to mention a few.
These are collectively known as hormone-transporting plasma proteins and will be discussed later in this article..
Types of Plasma Proteins
Albumin
As mentioned above, the albumin fraction is the most abundant and prominent band captured with electrophoresis.
Albumin is a single long polypeptide chain consisting of 610 amino acids. Albumin has two ends an N-terminal end and a C-terminal end. The N-terminal amino acid residue is aspartic acid, and the C-terminal residue is leucine. Serum albumin is made up of a large amount of essential amino acids such as arginine, histidine, and lysine, and the acidic amino acids aspartic acid and glutamic acid, as well as a small amount of tryptophan.
Approximately 50% of the albumin molecule shows an alpha-helical configuration, forming a doughnut shape.
Albumin is classified into two types depending on whether or not the free sulfhydryl group (S-H) is present. About two-thirds of blood serum albumin contains the free S-H group, and in the presence of Hg++, it tends to polymerize readily, forming a dimer. Therefore, it is called mercaptalbumin. The remaining third, which does not own a free S-H group, is non-mercaptalbumin, which has lost polymerizability.
The osmotic pressure of the blood is measured by the number of particles contained in a unit volume. The smaller the particles, the greater the resulting osmotic activity. Albumin’s molecular weight is relatively small, and its serum concentration is higher than other protein components. To be precise, 75% of blood colloid osmotic pressure is dependent on albumin, so it suffices to say that the osmotic pressure of the blood is determined primarily by albumin.
Globulins
As you may recall, alpha and beta globulins are transport proteins produced by the liver, whereas gamma globulins are part of the immune system and are produced by immune cells.
Alpha proteins are globular or spheric proteins that are highly active in alkaline or other electrically charged solutions. They typically have molecular weights of around 93 kDa. 3Alpha globulins consist of two principal fractions, α1 and α2.
The α1-fraction is second to the albumin fraction in its electrophoretic migration. It contains various serum protein components, among which are α1-antitrypsin, α1-acid glycoprotein, and α1-lipoprotein. The biological function of α1-antitrypsin is to inhibit the actions of trypsin and chymotrypsin. On the other hand, the function of α1-acid glycoprotein is still unknown, but it probably inhibits hemagglutination of the inactivated influenza virus and inactivates progesterone.
Similarly, the α2 fraction contains the major components of α2-macroglobulin, haptoglobin, and ceruloplasmin. Haptoglobin binds itself selectively to hemoglobin, while ceruloplasmin promotes oxidation and incorporates copper. By binding to proteolytic enzymes such as trypsin and plasmin, α2-macroglobulin causes them to lose their principal enzymatic activity.
Beta globulins are globular proteins in blood plasma that are relatively more mobile on an electrophoresis medium than gamma globulins but less mobile than alpha globulins.
The most critical component included in the β-fraction of globulins is transferrin. Among the β-globulins, transferrin is present in the greatest amount, representing approximately 50% of this fraction of globulins.
Transferrin binds to iron under physiological pH and forms an iron-transferrin complex. In fact, one-third of serum transferrin is bound to iron. The complement is part of the immune system, and its role is to form membrane-attacking complexes to kill pathogens, activate an inflammatory response, help phagocytosis, and neutralize toxins. Other essential components are hemopexin and properdin.
Gamma globulins or immunoglobulins are a functionally and structurally diverse group of proteins synthesized by plasma cells. First, they are biologically active, having characteristic antibody activity.
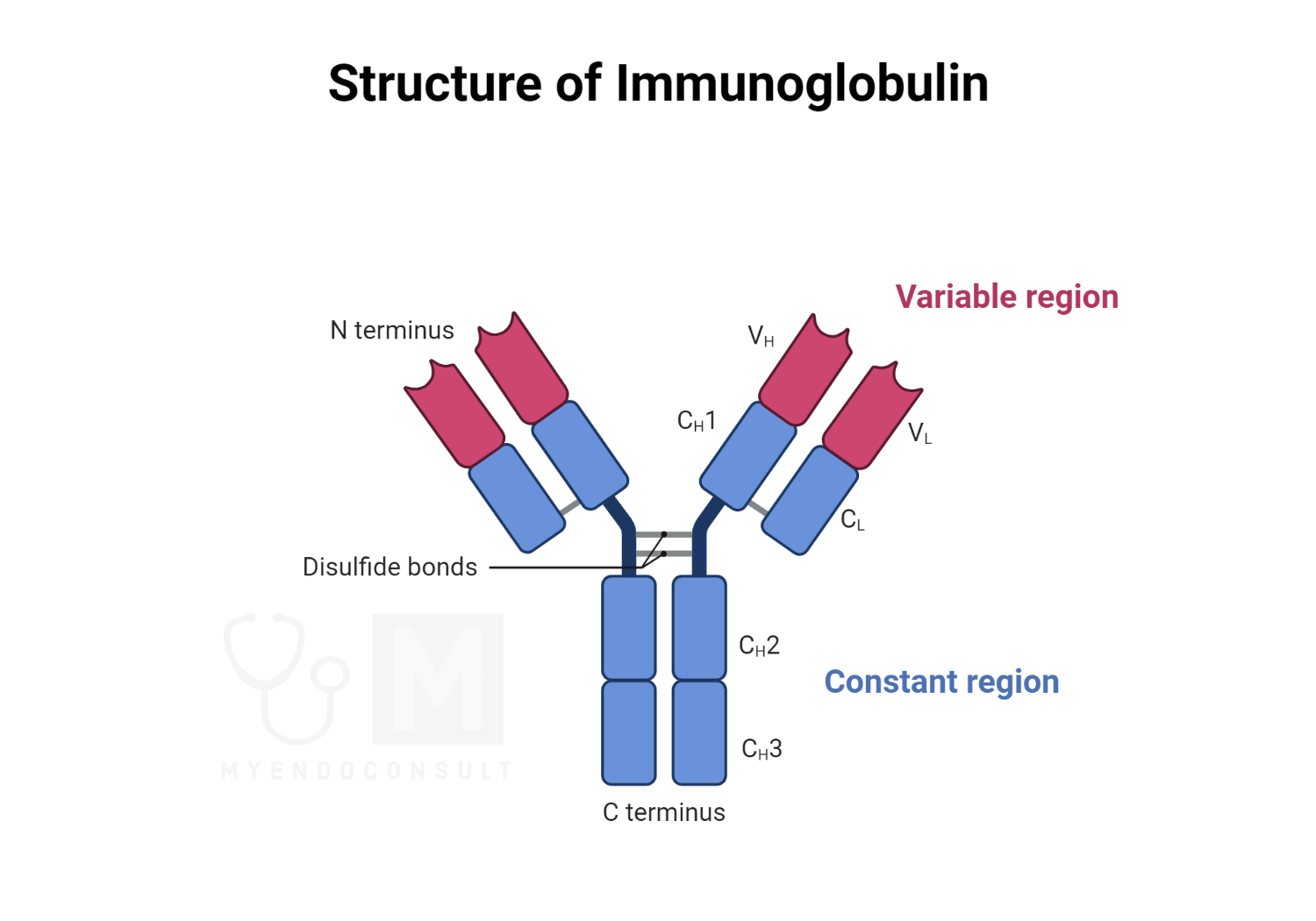
Figure 2.0 The structure of immunoglobulins
Second, they have a very similar chemical structures. The unit molecule of immunoglobulins is composed of four polypeptide chains held together by interchain disulfide and noncovalent bonds, including a pair of heavy polypeptide chains (H chains) and one pair of light polypeptide chains (L chains). The heavy chains, which have a molecular weight of approximately 60,000, are divided into five major classes γ, δ, α, μ, and ε depending on their antigenic specificity. The light chains, which have a molecular weight of approximately 23,000, are the same in all classes of immunoglobulins and are further divided into two types; κ (kappa) chain or K type and λ (lambda) chain or L type. Chains have variable and constant portions. The variable portions contribute to the binding affinity of an antibody globulin for its specific antigen, and their amino acid sequences vary one from another. The C-terminal segment, called the constant portion, has an essentially constant amino acid sequence for a given type of chain. On the basis of the five different heavy chains, there are five classes of Ig- IgM, IgA, IgG, IgE, and IgD.
Fibrinogen
Blood coagulation is a transformation of soluble fibrinogen molecules into a solid clot through a series of catalytic activities of various blood coagulation factors. Among the blood coagulation factors contained in plasma, fibrinogen is found in the largest amount, having the honor of being the Ist factor of coagulation. It migrates electrophoretically as the φ fraction between the β and γ fractions. Fibrinogen consists of 2,900 amino acids, composed of three pairs of different polypeptide chains: γ, β, and α.
Hormone-Transporting Plasma Proteins
Numerous substances that circulate in the bloodstream are bound to plasma proteins. Among these are antibiotics, vitamins, metal ions, lipids, and metabolic products such as bile pigments. Plasma proteins carry hormones such as gonadal steroids, adrenocorticoids, and thyroid hormones.
Approximately 97% of the female and male sex hormones in serum are bound to plasma proteins, especially albumin. Conjugated forms of glucuronides and sulfates are also bound to albumin.
Adrenocorticoids are mainly bound to transcortin and also to albumin, but less frequently. About 90-95% of adrenocorticoids in serum are bound to plasma proteins.
Furthermore, thyroid hormones bind to thyroxine-binding globulin, albumin, and prealbumin., and more than 99.9% of thyroxine exists in its bound form.
In the same way that calcium loses its physiological activity when bound to plasma proteins, hormones are also physiologically active in their free form, but they become inactive when bound to plasma proteins. This is one of the ways the endocrine system is controlled.
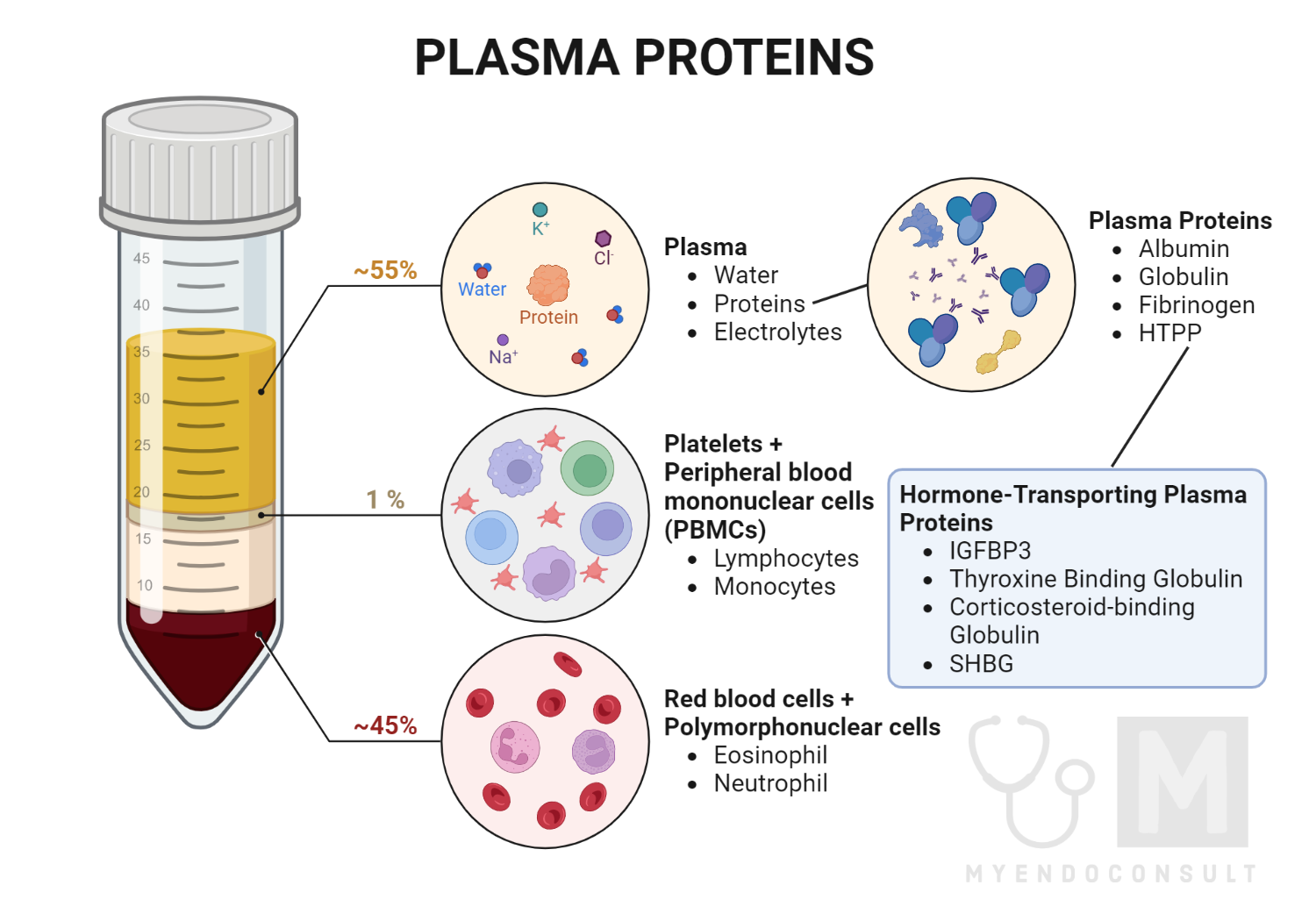
Figure 3.0 Components of plasma proteins
Measurement of Plasma Proteins
Plasma proteins can be separated and measured with many techniques. The most commonly used methods are agar or gel electrophoresis and different types of immunoassays.
Electrophoresis is a widely used laboratory technique that separates biomolecules, such as DNA, RNA, and proteins, based on their size and electrical charge. This technique is based on the principle that molecules with different sizes and charges will migrate through a matrix, such as a gel or a capillary tube, at different rates when subjected to an electric field.
In electrophoresis, the sample containing the molecules to be separated is applied to a gel or a capillary tube which acts as a sieve. An electric current is then applied to the gel or the tube, causing the molecules to migrate through the matrix. Smaller molecules will move more easily through the pores of the gel or tube and travel faster than larger molecules.
To determine the size of the molecules in a sample, standards of known sizes are separated on the same gel or tube and then compared to the sample. This allows researchers to estimate the size of the unknown molecules on their migration pattern relative to the known standards.
The protein sample is separated on a gel matrix and stained in protein electrophoresis to visualize the different protein bands. The main serum proteins, such as albumin, alpha1, alpha2, beta1, beta2, and gamma globulins, can be identified based on their migration patterns on the gel. Albumin is the largest peak and migrates closest to the positive electrode, while the globulin peaks migrate toward the negative electrode, with the gamma peak being closest to that electrode.
Electrophoresis is a powerful tool that is used in molecular biology, biochemistry, and clinical chemistry to separate and analyze different biomolecules based on their size and charge. It is widely used in research and diagnostic laboratories for a variety of applications, including the diagnosis of genetic diseases, the analysis of proteins and enzymes, and the detection of viral and bacterial infections.
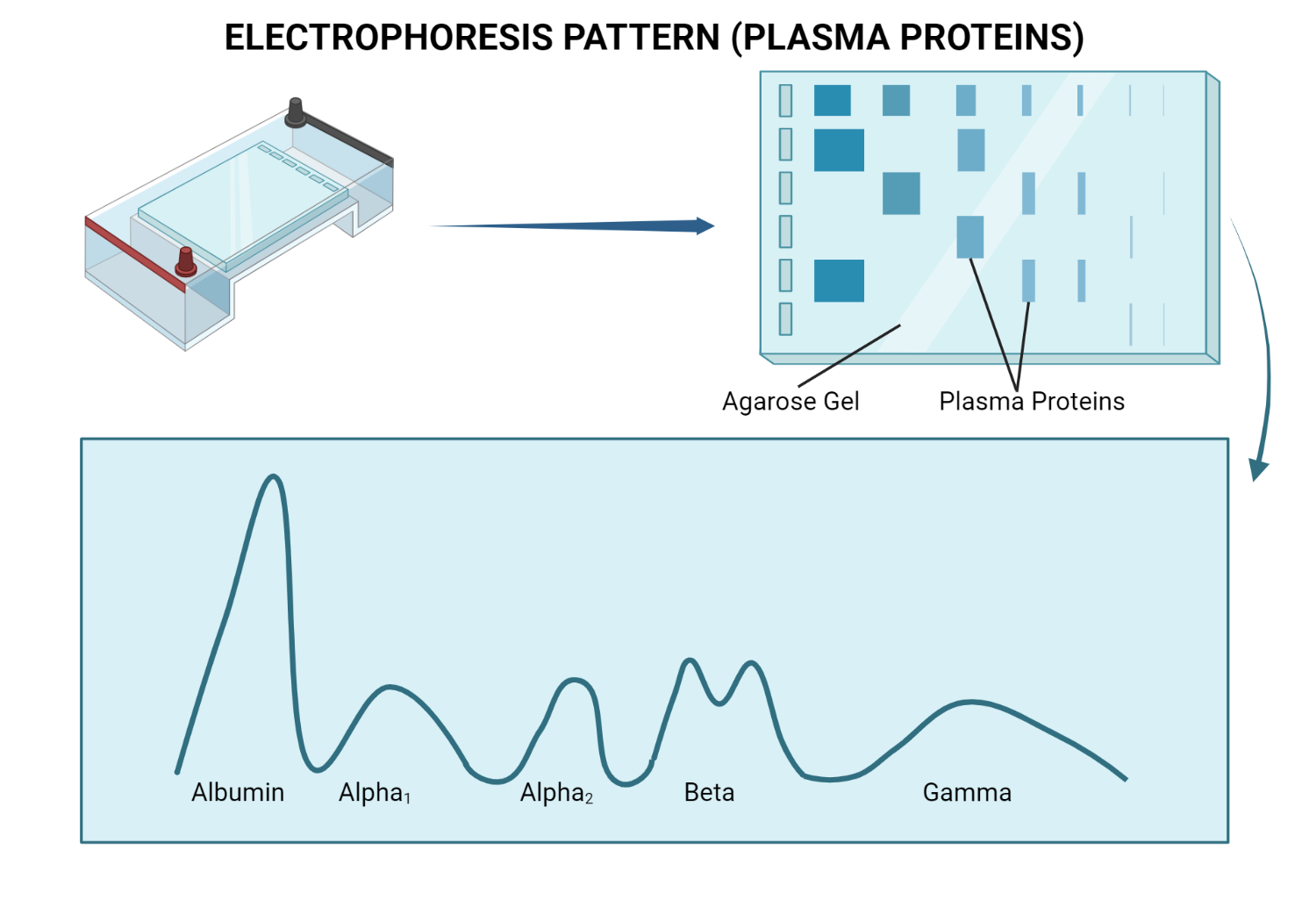
Figure 4.0 Electrophoresis pattern of plasma proteins
On the other hand, immunoassays detect the presence of a specific molecule in a sample using antibody-antigen binding reactions. Antibodies bind to the specific structure of a particular antigen, making immunoassays highly specific: the antibody will only bind to a specific structure of a particular antigen. This makes antibodies effective reagents for detecting target molecules. The most commonly used types of labeled immunoassays are Enzyme Immunoassay (EIA) or Enzyme-linked immunosorbent assay (ELISA), Radioimmunoassay (RIA), Fluoroimmunoassay (FIA), Chemiluminescence immunoassay (CLIA).
The results of the methods are significant in determining a diagnosis. For example, the presence of pathological immunoglobulins can be a sign of paraproteinemia. The increase of γ fraction can indicate liver damage, chronic inflammatory diseases, etc. Nephrotic syndrome, pregnancy, α-myelomas, malignancies, and acute inflammatory diseases can result in α fraction increase, etc.
Clinical importance of plasma proteins
Albumin
Since the liver produces albumin, logically, if a liver disease occurs, especially chronic one, there will be a change in albumin levels. The albumin fraction in serum tends to decrease, but decreases slightly only when liver damage is severe. However, in fulminant hepatitis, hepatocellular necrosis occurs widely, and the albumin fraction eventually decreases due to a significant deficiency of albumin synthesis.
In nephrotic syndrome, large amounts of plasma proteins are excreted into the urine. A decrease in albumin fraction is inevitable, and generalized edema always occurs when this drops below 2 g/100 ml. Although a decrease in serum albumin is observed, prealbumin tends to increase. On the other hand, there is a decrease in the albumin turnover rate in renal failure, which distinguishes these conditions.
If the blood amino acid pool is deficient, protein synthesis decreases even if the synthesized cells have their normal functions, as could occur in pathological states such as malnutrition, cachexia, and starvation. Among them, cachexia and Marasmus-Kwashiorkor show the most severe state of malnutrition, with a rapid decrease in intravascular and extravascular albumin pools. The main reason is the lack of nutrients, which decreases the synthetic and catabolic rate of albumin.
Globulins
Since immunoglobulins are antibodies that bind antigens, their function is related to protecting our body from pathogens.
For example, when the body becomes hypersensitive to antigenic stimulation, there is an abnormal increase in immunoglobulin synthesis. Chronic infections where the antigen cannot be removed also cause a constant production of immunoglobulins. This mechanism is also involved in autoimmune diseases, but the antigen is part of our tissues.
Lastly, multiple myeloma is a type of bone cancer in which anaplastic plasma cells synthesize pathological M proteins instead of normal antibodies. In addition, normal immunoglobulins tend to decrease, so the patient’s immune system weakens. It is observed that the greater the number of M proteins, the stronger the tendency to see a decrease in normal immunoglobulins.
Fibrinogen
Fibrinogen is a protein produced by the liver that plays an essential role in blood clotting. Elevated fibrinogen levels have been associated with cardiovascular disease, including coronary, cerebral, and peripheral atherosclerosis. Fibrinogen can contribute to atherosclerosis by promoting platelet aggregation, increasing plasma viscosity, and improving blood clots.
Prospective studies have found that fibrinogen is an independent predictor of myocardial infarction in both sexes. However, it is unclear whether elevated fibrinogen levels are a cause or a consequence of cardiovascular disease because no drugs selectively lower plasma fibrinogen levels are available.
In addition to its role in cardiovascular disease, fibrinogen has recently been shown to play vital role in tumorigenesis. Elevated plasma fibrinogen levels are associated with tumor progression and poor outcomes in several types of malignancies, including covered esophageal cancer, gastric cancer, pancreatic cancer, colon cancer, lung cancer, hepatocellular cancer, gallbladder cancer, and gynecological cancer.
For example, elevated preoperative plasma fibrinogen levels are associated with cancer progression and prognosis in operable breast cancer patients. Fibrinogen has been shown to contribute to angiogenesis, stromal formation, and hematogenous metastasis of tumor cells.
Elevated plasma fibrinogen levels are associated with several health conditions, including cardiovascular disease and cancer. More research is needed to determine the causal relationship between fibrinogen and these conditions and to develop targeted therapies to lower plasma fibrinogen levels.
Hormone-Transporting Plasma Proteins
HTPPs regulate the amount of hormone reaching target cells and help stabilize endocrine system homeostasis, releasing only the amount of hormones needed. Some pathological states can cause changes in these proteins, leading to significant changes in free and bound hormones.
Sex hormone-binding globulin (SHBG) is a glycoprotein with a high affinity for hormones such as testosterone and estradiol. It is synthesized in the liver, and its plasma concentration is regulated by androgen/estrogen balance, thyroid hormones, insulin, and dietary factors. SHBG is involved in the transport of sex steroids in the plasma, and its concentration is a significant factor in the regulation of their distribution between the protein-bound and free states.
Plasma SBHG concentrations are affected by many diseases, such as hyperthyroidism, hypogonadism, pregnancy, anorexia nervosa, androgen insensitivity, and liver cirrhosis, which results in high concentrations. On the other hand, low concentrations are found in myxoedema, hyperprolactinemia, syndromes of excessive androgen activity (polycystic ovary disease), obesity, diabetes, Cushing syndrome, and acromegaly.
Of the steroid hormones, corticosteroids are also bound to carrier proteins such as transcortin or corticosteroid-binding globulin (CBG). Approximately two-thirds of the cortisol in normal serum binds to CBG. It can bind to other adrenocorticosteroids such as corticosterone, aldosterone, and progesterone. Hypoproteinemia, Cushing syndrome, corticoid treatments, vitamin B12 deficiency, and septic shock decrease CBG production.
Next, we have thyroxine-binding globulin, which tends to bind specifically to thyroxine, and two-thirds of the thyroxine in normal serum is bound to it. It also binds to triiodothyronine, but thyroxine has an affinity about three times stronger. Acute intermittent porphyria, HIV, severe liver disease, hypothyroidism, and pregnancy increase TBG production. In contrast, kidney failure, acute illness, acromegaly, hyperthyroidism, and malnutrition cause a decrease in production.
Meanwhile, insulin-like growth factors (IGFs) are essential growth-promoting peptides that act as both endocrine hormones and autocrine/paracrine growth factors. In the bloodstream and local tissues, most IGF molecules are bound by one of the members of the IGF-binding protein (IGFBP) family, of which there are six different types. Binding to an IGFBP increases the half-life of the IGF in circulation and blocks its potential binding to the insulin receptor.
Vitamin D binding protein, the transporter of vitamin D and its metabolites in the blood, is recognized to be a member of a gene family that includes albumin and alpha-fetoprotein. Identical to the group-specific component (Gc-globulin) of serum, the protein is a constitutively synthesized single-chain polypeptide in the liver that circulates in amounts far in excess of normal vitamin D metabolites in blood. It plays a significant role in the egress of endogenously synthesized vitamin D from the skin and appears to restrict D-sterols from too rapid/excessive cell entry.
Finally, alpha-fetoprotein (AFP) is a product of specific fetal tissues and neoplastic cells of hepatocyte or germ cell origin in adults. This protein belongs to a family that is phylogenetically most closely related to serum albumin. AFP, like serum albumin, shows strong binding affinities for a variety of ligands. The most notable difference is the strong preferential binding of AFPs of polyunsaturated fatty acids. This protein may play a role in transporting these substances in the period of development and in the growth of malignant cells. Various agents affect the synthesis of this protein, both by specific fetal tissues and by neoplastic cells. Marked differences in cell responses, particularly those of neoplastic types, indicate variations in the genetic factors responsible for the control of their synthesis.
In conclusion, plasma proteins are crucial components of human blood that serve many essential functions. From regulating blood viscosity and osmotic pressure to defending against infections and transporting nutrients, these proteins are vital for maintaining overall health and well-being. One of the most significant functions of plasma proteins is to transport hormones throughout the body. Hormones play a crucial role in regulating various physiological functions, and without hormone-transporting plasma proteins, they would not be able to reach their intended targets. The importance of plasma proteins in hormone transport cannot be overstated, as they are critical for the proper functioning of the human body.
Selected References
- Peters, T. Intracellular precursor forms of plasma proteins: their functions and possible occurrence in plasma. Clin Chem. 1987 Aug;33(8):1317-25.
- Heinrich J, Assmann G. Fibrinogen and cardiovascular risk. J Cardiovasc Risk. 1995 Jun;2(3):197-205.
- Selby C. Sex hormone binding globulin: origin, function, and clinical significance. Ann Clin Biochem. 1990 Nov;27 ( Pt 6):532-41
- Deutsch HF. Chemistry and biology of the alpha-fetoprotein. Adv Cancer Res. 1991;56:253-312.
- Sandor, G. (1966). Serum proteins in health and disease.
Kindly Let Us Know If This Was helpful? Thank You!


