The mechanism of action of somatostatin analog therapy and its clinical application in managing acromegaly will be reviewed.
Physiology
The role of somatostatin in growth hormone regulation: Growth hormone-releasing hormone (GHRH) is a peptide hormone composed of 44 amino acids synthesized in the arcuate nucleus of the hypothalamus. GHRH is secreted in a pulsatile fashion and is carried from the hypothalamus to the anterior pituitary gland via the hypothalamo-hypophyseal vessels. By binding to receptors on the surface of anterior pituitary somatotrophs, GHRH stimulates gene transcription, translation, and the eventual release of growth hormone (GH) from their secretory vesicles. Somatostatin (also known as somatostatin receptor inhibitory factor, SRIF), on the other hand, blocks the release of GH by somatotrophs by acting on somatostatin receptors (primarily SSR2 receptors)(1). Ghrelin (derived from gastric oxyntic cells) is a GH secretagogue that exhibits its effects by acting on hypothalamic GHRH cells in the median eminence(2). GH and insulin-like growth factor 1 (IGF-1) provide additional negative feedback inhibition of GH secretion (see figure 1.0).
Table 1.0 Regulators of circulating levels of GH
| Promotes GH secretion | Inhibits GH secretion |
| Arginine (3) | Overnutrition(4) |
| Clonidine (3) | Glucocorticoids (high doses)(5) |
| Estrogen Δ (6) | Somatostatin (1) |
| Hypoglycemia, Glucagon (7) | |
| L-dopa(3) | |
| Exercise(3) |
Δ This effect is route-dependent, with the oral route causing an increase in GH and a paradoxically low IGF-1. Transdermal estrogen does not produce this effect(6).
Adapted from references (1,3–7)
GH binds to the extracellular component of the hepatic GH receptor (GH-R) and induces a series of intracellular processes required for the transcription and translation of specific genes that encode insulin-like growth factor 1 (IGF1), IGF-binding protein 3 (IGFBP3), and an acid-labile subunit (ALS)(8–10). These products of GH action at the level of the liver form a ternary complex in circulation that influences the ability of IGF-1 to bind its peripheral insulin-like growth factor 1 receptor (IGF-1R). Indeed, posttranslational modification of IGFBP3 (e.g., glycosylation, phosphorylation) is an essential determinant of IGF-1’s ability to bind the IGF-1R. For example, glycosylation of IGFBP3 increases the ability of IGF-1 to bind IGF-1R. Deglycosylation, on the other hand, impairs ligand-to-receptor binding(11). The consequences of GH action are widely accepted as pivotal for macronutrient (carbohydrate, lipid, and protein) metabolism and cellular growth(12).
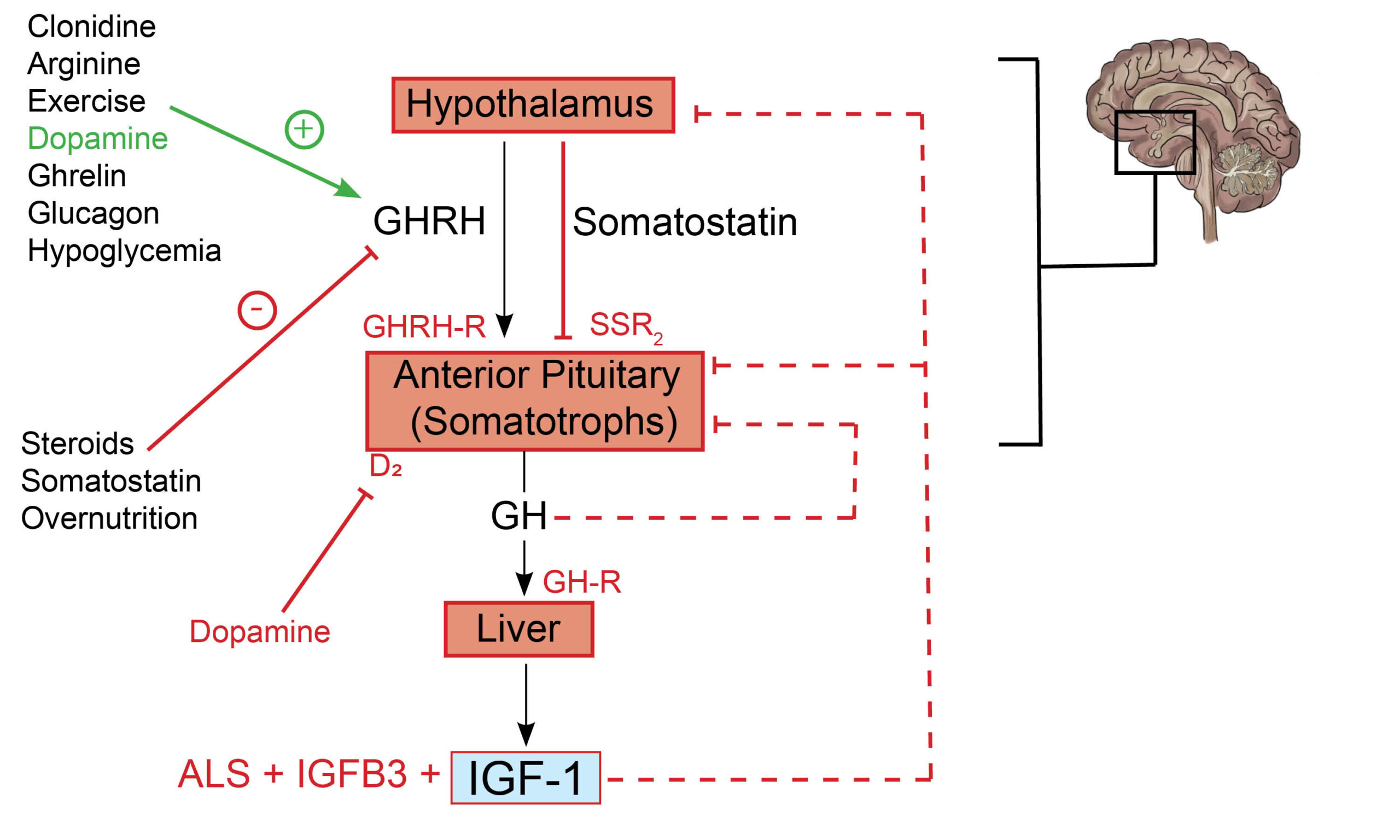
Fig. 1.0 The growth hormone and insulin-like growth factor 1 axis. Hypothalamic-derived GHRH binds to GHRH-R on anterior pituitary somatotrophs and consequently mediates their secretion of GH. The binding of GH to GH-R in the liver promotes the synthesis of IGF-1, ALS, and IGFB3 – a complex that determines the circulating half-life and, thus, peripheral effects of IGF-1. IGF-1 exerts negative feedback inhibition of GH and GHRH at the anterior pituitary and hypothalamus, respectively (dashed arrow). GH exerts negative feedback inhibitory effects on its production at the level of the anterior pituitary gland (dashed arrow). Somatostatin inhibits the release of growth hormone by somatotrophs. Important co-inhibitory and co-stimulatory factors influencing net GH secretion are shown (negative and positive signs). Redrawn and modified from Ranke MB, Wit JM (2018) Growth hormone – past, present and future. Nat Rev Endocrinol 14:285–300
Mechanism of action
Somatostatin analogs (SSAs) activate somatostatin receptors on somatotroph tumor cells and thus inhibits their production of GH and, consequently, hepatic IGF-1. There is evidence that SSAs lead to a reduction in the size of GH secreting tumors as well(13,14).
Practice Guide
SSAs approved in the United States for the management of acromegaly include octreotide (short release), octreotide long-acting release (Sandostatin LAR), and lanreotide (Somatuline). Also review the use of pasireotide in Cushing’s disease.
- A hallmark side effect of pasireotide is hyperglycemia. The risk of hyperglycemia is disproportionately higher among patients with either diabetes or prediabetes; it is, therefore, reasonable to screen for hyperglycemia before and during treatment (15).
- First-generation SSAs (for example, lanreotide and octreotide) have a greater affinity for SSR2 than the SSR5 isoform of the somatostatin receptor(16). Pasireotide, on the other hand, has a greater affinity for the SSR5 receptor subtype. The response of tumors is influenced by the density of specific histologic SSR subtypes (e.g., sparsely vs. densely granulated growth hormone-secreting tumors)(17). This is clinically relevant since it can influence the effectiveness of selected therapies. For instance, sparsely granulated somatotroph tumors tend to express SSR5 receptors (pasireotide). Densely granulated somatotrophs, on the other hand, express predominantly the SSR2 subtype(octreotide)(17–19).
- Somatostatin analogs are associated with an increased risk of cholelithiasis and diarrhea(20).
- Monitoring response to therapy – IGF-1 should normalize to the age and gender-specific reference range for IGF-1. Also, GH should suppress to a nadir of <1 ng/mL or <0.4ng/mL (for newer, more sensitive GH assays) after an oral glucose load with 75grams of anhydrous glucose (Oral glucose tolerance test).
Clinical Trial Evidence
Pasireotide, a second-generation multi-receptor SSA, causes a more significant biochemical amelioration of acromegaly in contrast to first-generation SSAs (octreotide and lanreotide) (21,22).
The PAOLA study (Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly) was a randomized, prospective, parallel-group study evaluating the safety and efficacy of pasireotide. A total of 198 patients with uncontrolled acromegaly (GH >2.5mcg/L and IGF >1.3 times the age and gender-adjusted upper limit of normal) were on either octreotide or lanreotide for a minimum of 6 months.
Patients were randomized to pasireotide (40mg or 60mg) administered intramuscularly once every 28days or active comparators (octreotide or lanreotide). The study authors defined the primary outcome as GH <2.5mcg/L and normalization of IGF-1. The primary outcome occurred in 15% (40mg), 20% (60mg) and 0% (octreotide/lanreotide)(21)
References
- Blum WF, Alherbish A, Alsagheir A, El Awwa A, Kaplan W, Koledova E, et al. The growth hormone–insulin-like growth factor-I axis in the diagnosis and treatment of growth disorders. Endocr Connect. 2018 May 3;7(6):R212–22.
- Anderson LL, Scanes CG. Nanobiology and physiology of growth hormone secretion. Exp Biol Med. 2012 Feb;237(2):126–42.
- de Fátima Borges M, Teixeira FCC, Feltrin AK, Ribeiro KA, Nascentes GAN, Resende EAMR, et al. Clonidine-stimulated growth hormone concentrations (cut-off values) measured by immunochemiluminescent assay (ICMA) in children and adolescents with short stature. Clinics. 2016 Apr;71(4):226–31.
- Fazeli PK, Klibanski A. Determinants of Growth Hormone Resistance in Malnutrition. J Endocrinol. 2014 Jan 27;220(3):R57–65.
- Mazziotti G, Giustina A. Glucocorticoids and the regulation of growth hormone secretion. Nat Rev Endocrinol. 2013 May;9(5):265–76.
- Leung KC, Johannsson G, Leong GM, Ho KKY. Estrogen regulation of growth hormone action. Endocr Rev. 2004 Oct;25(5):693–721.
- Hawkes CP, Grimberg A, Dzata VE, De Leon DD. Adding Glucagon-Stimulated GH Testing to the Diagnostic Fast Increases the Detection of GH-Sufficient Children. Horm Res Paediatr. 2016;85(4):265–72.
- Donaghy AJ, Delhanty PJD, Ho KK, Williams R, Baxter RC. Regulation of the growth hormone receptor/binding protein, insulin-like growth factor ternary complex system in human cirrhosis. J Hepatol. 2002 Jun;36(6):751–8.
- Domené HM, Hwa V, Jasper HG, Rosenfeld RG. Acid-labile subunit (ALS) deficiency. Best Pract Res Clin Endocrinol Metab. 2011 Feb;25(1):101–13.
- Poyrazoğlu Ş, Hwa V, Baş F, Dauber A, Rosenfeld R, Darendeliler F. A Novel Homozygous Mutation of the Acid-Labile Subunit (IGFALS) Gene in a Male Adolescent. J Clin Res Pediatr Endocrinol. 2019 Dec;11(4):432–8.
- Ranke MB, Wit JM. Growth hormone – past, present and future. Nat Rev Endocrinol. 2018;14(5):285–300.
- Devesa J, Almengló C, Devesa P. Multiple Effects of Growth Hormone in the Body: Is it Really the Hormone for Growth? Clin Med Insights Endocrinol Diabetes. 2016 Oct 12;9:47–71.
- Carmichael JD, Bonert VS, Nuño M, Ly D, Melmed S. Acromegaly Clinical Trial Methodology Impact on Reported Biochemical Efficacy Rates of Somatostatin Receptor Ligand Treatments: A Meta-Analysis. J Clin Endocrinol Metab. 2014 May;99(5):1825–33.
- Gariani K, Meyer P, Philippe J. Implications of Somatostatin Analogues in the Treatment of Acromegaly. Eur Endocrinol. 2013 Aug;9(2):132–5.
- McKeage K. Pasireotide in Acromegaly: A Review. Drugs. 2015 Jun;75(9):1039–48.
- Dineen R, Stewart PM, Sherlock M. Acromegaly. QJM Int J Med. 2017 Jul 1;110(7):411–20.
- Iacovazzo D, Carlsen E, Lugli F, Chiloiro S, Piacentini S, Bianchi A, et al. Factors predicting pasireotide responsiveness in somatotroph pituitary adenomas resistant to first-generation somatostatin analogues: an immunohistochemical study. Eur J Endocrinol. 2016 Feb;174(2):241–50.
- Mayr B, Buslei R, Theodoropoulou M, Stalla GK, Buchfelder M, Schöfl C. Molecular and functional properties of densely and sparsely granulated GH-producing pituitary adenomas. Eur J Endocrinol. 2013 Oct 1;169(4):391–400.
- Amarawardena WKMG, Liyanarachchi KD, Newell-Price JDC, Ross RJM, Iacovazzo D, Debono M. Pasireotide: successful treatment of a sparsely granulated tumour in a resistant case of acromegaly. Endocrinol Diabetes Metab Case Rep [Internet]. 2017 Jul 10 [cited 2021 Apr 24];2017. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5510455/
- Gomes-Porras M, Cárdenas-Salas J, Álvarez-Escolá C. Somatostatin Analogs in Clinical Practice: a Review. Int J Mol Sci. 2020 Feb 29;21(5).
- Gadelha MR, Bronstein MD, Brue T, Coculescu M, Fleseriu M, Guitelman M, et al. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomized, phase 3 trial. Lancet Diabetes Endocrinol. 2014 Nov;2(11):875–84.
- Gadelha M, Bex M, Colao A, Pedroza García EM, Poiana C, Jimenez-Sanchez M, et al. Evaluation of the Efficacy and Safety of Switching to Pasireotide in Patients With Acromegaly Inadequately Controlled With First-Generation Somatostatin Analogs. Front Endocrinol. 2020;10:931.
Explore the pathophysiology of various endocrine diseases and the mechanism of action of medications utilized in their treatment. Click here to learn more!
Kindly Let Us Know If This Was helpful? Thank You!


