Cinacalcet is a calcimimetic agent utilized in the treatment of primary hyperparathyroidism, parathyroid cancer and secondary hyperparathyroidism. Osteitis fibrosa cystica also known as “von Recklingshausen's disease of bone” was first reported in 1891[1]. However, the association between this condition and a parathyroid tumor was clearly demonstrated by Dr. Felix Mandl in 1925, after removal of a parathyroid tumor in a patient with radiographic features consistent with OFC, resulted in a dramatic resolution of hypercalciuria and bone pain[2].
Primary hyperparathyroidism (PHPT) is a cause of hypercalcemia and has a highly variable reported incidence rate. However, there are no population-based estimates of the prevalence of this condition[3].
It is characterized by hypercalcemia in the setting of elevated or inappropriately normal serum intact parathyroid hormone. Autonomous parathyroid hormone production either in a single parathyroid adenoma or multiple adenomas accounts for manifestations of this condition. Kidney stones, fragility fractures (osteoporosis), and symptomatic hypercalcemia are known complications.
The indications for surgical treatment in primary hyperparathyroidism include the following, age <50years, serum calcium greater than 1mg/dl above the upper limit of normal, osteoporosis, estimated glomerular filtration rate <60 ml/min/1.73m2, and nephrolithiasis/urolithiasis[4].
Medical therapy may serve as a bridge to surgery or a reasonable alternative to surgery in special circumstances. For example, patients not deemed optimal surgical candidates may be managed with medical therapy(5).
Cinacalcet, a calcimimetic agent, lowers serum calcium in patients with hypercalcemia in the setting of primary hyperparathyroidism. The basic pathophysiology underlying the mechanism of action of cinacalcet will be reviewed in this article.
Physiology
Regulation of serum calcium and phosphorus
The regulation of serum calcium and phosphorus is dependent on an intricate relationship between parathyroid hormone, 1α,25 dihydroxyvitamin D (1α,25(OH)2D), and fibroblast growth factor 23. The skeleton, gastrointestinal tract, and kidney are the principal sites of action of these key regulators of serum calcium and phosphorus(6).
The skeleton serves as an extensive repository of total body calcium and phosphorus. It is worth noting that bone comprises calcium and phosphorus-containing hydroxyapatite crystals (Ca10(PO4)6(OH)2), collagenous and noncollagenous proteins. Indeed, approximately 99% (about 1kilogram in a healthy adult)(7) and 85% (about 700grams) of total body calcium and phosphorus are present in bone, respectively(7,8).
Consequently, only 0.1% of calcium and about 1% of phosphorus exist in the extracellular fluid compartment(7). Parathyroid hormone facilitates calcium reabsorption and phosphate excretion in the kidneys. PTH is also critical in the hydroxylation of 25 hydroxyvitamin D (25-OHD) at its 1α position, resulting in calcitriol formation (1α,25(OH)2D).
Calcitriol subsequently promotes intestinal calcium and phosphate conservation (see section 5.2.2) (9). Additionally, PTH indirectly activates osteoclasts responsible for the liberation of calcium and phosphorus from the skeleton (10).
Fibroblast growth factor 23 (FGF23) inhibits both renal phosphate conservation and calcitriol formation, which results in a net effect of a reduced level of serum calcium and phosphorus (11)
Calcitonin, a less characterized hormone in calcium physiology, reduces calcium resorption from skeletal stores and also inhibits renal reabsorption of calcium (12). This explains the utility of calcitonin in the acute treatment of hypercalcemia (13).
The Calcium-PTH Curve: A Dynamic Physiologic Response
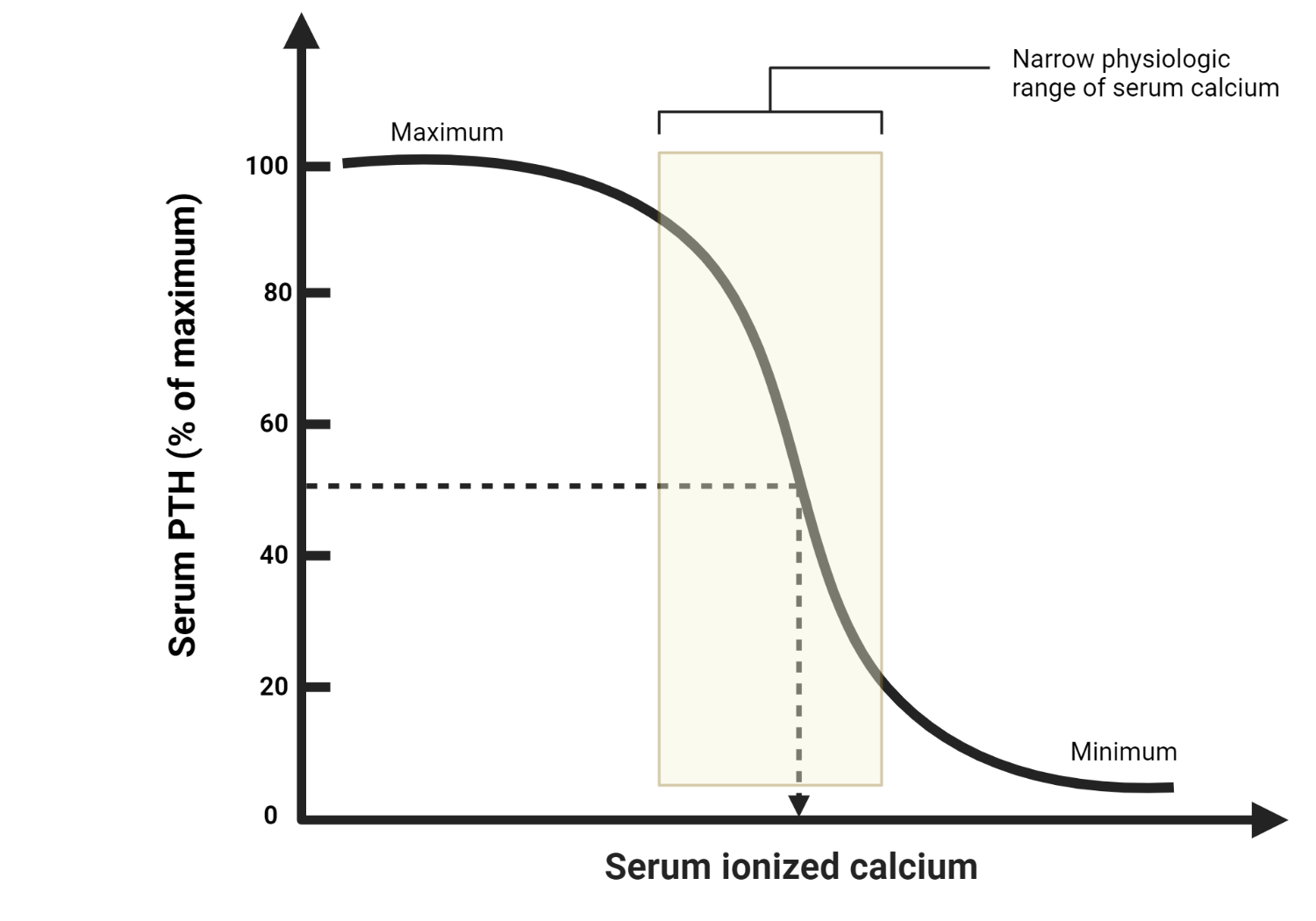
The relationship between calcium and PTH is commonly represented as a sigmoidal curve, often referred to as the “calcium-PTH curve” or “PTH set-point curve.” On the X-axis of this curve, we have the serum calcium concentration, while the Y-axis represents the serum PTH levels.
The sigmoidal shape of the curve implies that small changes in serum calcium levels result in significant alterations in PTH secretion when calcium concentrations are near or below the normal range. On the other hand, when calcium concentrations are within or above the normal range, changes in PTH secretion are less pronounced
Calcium sensing and PTH regulation
Calcium is required for various bodily functions, including hormonal secretion, muscle contraction, coagulation, neural transmission, to mention a few(14,15). Calcium exists in several forms in extracellular fluid. These include its free or ionized form (about 50% of circulating calcium), albumin-bound (~40%), and complexed form (~10% bound to anions such as bicarbonate, citrate, phosphates, and citrate)(16).
The calcium-sensing receptor (CaSR), a G-protein coupled receptor expressed by the chief cells of the parathyroid gland, C cells of the thyroid, and renal tubules, plays a pivotal role in regulating serum calcium. The activation of CaSR blunts the synthesis and eventual release of PTH by the parathyroids, augments calcitonin release by C cells of the thyroid, and finally inhibits renal calcium reabsorption (independent of PTH action).
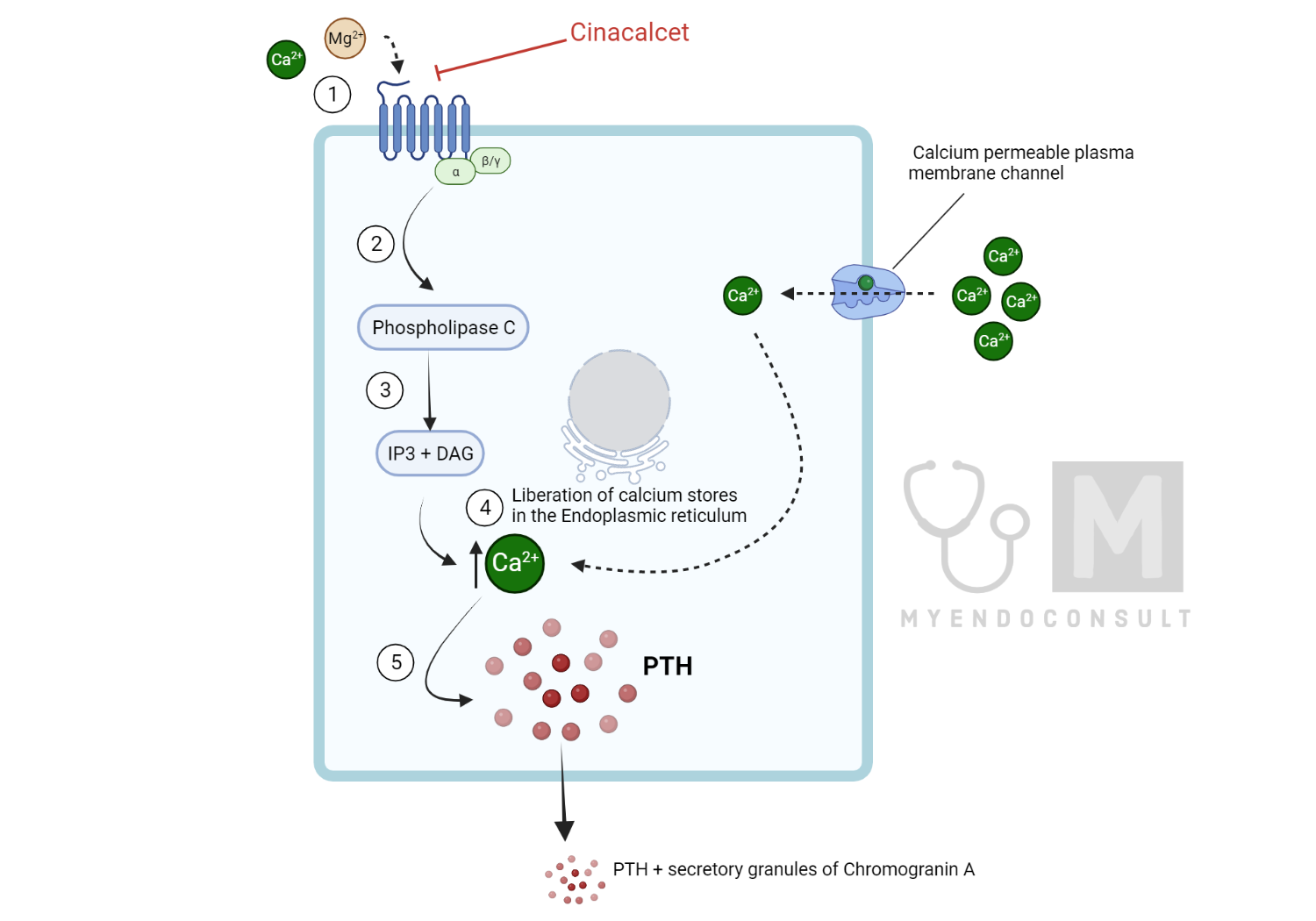
At the level of the parathyroid glands, CaSR’s activation by ionized calcium (an extracellular first messenger) results in downstream processes (Phospholipase C-Inositol triphosphate-diacylglycerol pathway), which increases the liberation of calcium from its stores in the endoplasmic reticulum(17,18). Increased intracellular calcium inhibits the fusion of PTH-containing vesicles with the plasma membrane, which results in reduced secretion of PTH. Additionally, the transcription and translation of PTH are regulated by 1α,25(OH)2D (binding of active vitamin D to vitamin D response elements in the promoter region of the PTH gene promotes PTH synthesis)(not shown)(19,20). Similarly, magnesium, another relevant extracellular divalent cation, can also activate the CaSR and impair PTH synthesis(17).
Mechanism of Action of Cinacalcet
Calcimimetics promote the sensitivity of the CaSR to serum calcium by lowering the set point for activation of the receptor. This, in effect, leads to the activation of the CaSR even at lower levels of ionized calcium, a process that inhibits PTH release(21).
In contrast to divalent cations (calcium and magnesium), which bind the amino-terminal domain of the CaSR, calcimimetics bind to the 7-transmembrane domain of the receptor (see figure 5.1). Calcimimetics promote a conformational change in the CaSR, resulting in increased receptor sensitivity to extracellular calcium (a positive allosteric effect)(22,23).
Ultimately, these agents reduce serum calcium by inhibiting PTH secretion and increasing renal calcium excretion(23). Cinacalcet is a prototypical calcimimetic utilized in the medical management of primary hyperparathyroidism(4,5).
Practice Pearl(s)
- Serum calcium should be checked within a week of a dose change of cinacalcet (sensipar).
- Hypotension and arrhythmias are possible severe reactions. Nausea and diarrhea are, however, more likely side effects (24).
- Indications for cinacalcet use in PHPT include poor surgical candidates (or refuse surgery) and those with hypercalcemia refractory to curative parathyroidectomy(25).
Clinical Trial Evidence
Cinacalcet is FDA-approved for the management of significant hypercalcemia in primary hyperparathyroidism.
Cinacalcet can lead to >1mg/dL decline in serum calcium from baseline in patients with primary hyperparathyroidism.
In this phase three, double-blind, multicenter randomized placebo-controlled trial, 140 subjects were randomized to either cinacalcet or placebo. Inclusion criteria included total corrected serum calcium between 11.3 and 12.5mg/dl, failed parathyroidectomy, or poor surgical candidates. The primary outcome (normalization of serum calcium) was assessed at 28weeks, after which subjects in both arms were enrolled in an open-label extension phase of the study. The primary outcome occurred in 84.8% of subjects in the intervention arm and 5.9% in the placebo arm. This was statistically significant. Interestingly, adverse events (nausea and muscle spasms) were similar between both arms of the study(26).
References
1. Misiorowski W, Bilezikian JP. Osteitis Fibrosa Cystica. JBMR Plus. 2020 Sep 7;4(9):e10403.
2. Mandl F. Hyperparathyroidism; a review of historical developments and the present state of knowledge on the subject. Surgery. 1947 Mar;21(3):394–440.
3. Clarke BL. Epidemiology of Primary Hyperparathyroidism. J Clin Densitom. 2013 Jan 1;16(1):8–13.
4. Bilezikian JP. Primary Hyperparathyroidism. J Clin Endocrinol Metab. 2018 Nov 1;103(11):3993–4004.
5. Ng CH, Chin YH, Tan MHQ, Ng JX, Yang SP, Kiew JJ, et al. Cinacalcet and primary hyperparathyroidism: systematic review and meta regression. Endocr Connect. 2020 Jul;9(7):724–35.
6. Felsenfeld A, Rodriguez M, Levine B. New insights in regulation of calcium homeostasis. Curr Opin Nephrol Hypertens. 2013 Jul;22(4):371–6.
7. Moorthi RN, Moe SM. CKD–Mineral and Bone Disorder: Core Curriculum 2011. Am J Kidney Dis Off J Natl Kidney Found. 2011 Dec;58(6):1022–36.
8. Vorland CJ, Stremke ER, Moorthi RN, Hill Gallant KM. Effects of Excessive Dietary Phosphorus Intake on Bone Health. Curr Osteoporos Rep. 2017 Oct;15(5):473–82.
9. Adams JS, Hewison M. Update in Vitamin D. J Clin Endocrinol Metab. 2010 Feb 1;95(2):471–8.
10. Summers R, Macnab R. Thyroid, parathyroid hormones and calcium homeostasis. Anaesth Intensive Care Med. 2017 Oct 1;18(10):522–6.
11. Martin A, David V, Quarles LD. Regulation and Function of the FGF23/Klotho Endocrine Pathways. Physiol Rev. 2012 Jan 1;92(1):131–55.
12. Davey RA, Findlay DM. Calcitonin: physiology or fantasy? J Bone Miner Res Off J Am Soc Bone Miner Res. 2013 May;28(5):973–9.
13. Felsenfeld AJ, Levine BS. Calcitonin, the forgotten hormone: does it deserve to be forgotten? Clin Kidney J. 2015 Apr;8(2):180–7.
14. Pu F, Chen N, Xue S. Calcium intake, calcium homeostasis and health. Food Sci Hum Wellness. 2016 Mar 1;5(1):8–16.
15. Bagur R, Hajnóczky G. Intracellular Ca2+ sensing: role in calcium homeostasis and signaling. Mol Cell. 2017 Jun 15;66(6):780–8.
16. Bushinsky DA, Monk RD. Calcium. The Lancet. 1998 Jul 25;352(9124):306–11.
17. Tfelt-Hansen J, Brown EM. The calcium-sensing receptor in normal physiology and pathophysiology: a review. Crit Rev Clin Lab Sci. 2005;42(1):35–70.
18. Goodman HM. Chapter 10 – Hormonal Regulation of Calcium Balance. In: Goodman HM, editor. Basic Medical Endocrinology (Fourth Edition) [Internet]. San Diego: Academic Press; 2009. p. 197–218. Available from: https://www.sciencedirect.com/science/article/pii/B9780123739759000100
19. Kumar R, Thompson JR. The regulation of parathyroid hormone secretion and synthesis. J Am Soc Nephrol JASN. 2010/12/16 ed. 2011 Feb;22(2):216–24.
20. Goltzman D, Mannstadt M, Marcocci C. Physiology of the Calcium-Parathyroid Hormone-Vitamin D Axis. Front Horm Res. 2018;50:1–13.
21. Pereira L, Meng C, Marques D, Frazão JM. Old and new calcimimetics for treatment of secondary hyperparathyroidism: impact on biochemical and relevant clinical outcomes. Clin Kidney J. 2018 Feb;11(1):80–8.
22. Jensen AA, Bräuner-Osborne H. Allosteric Modulation of the Calcium-Sensing Receptor. Curr Neuropharmacol. 2007 Sep;5(3):180–6.
23. Nemeth EF. Allosteric modulators of the extracellular calcium receptor. Drug Discov Today Technol. 2013;10(2):e277-284.
24. Luque-Fernández I, García-Martín A, Luque-Pazos A. Experience with cinacalcet in primary hyperparathyroidism: results after 1 year of treatment. Ther Adv Endocrinol Metab. 2013 Jun;4(3):77–81.
25. Marcocci C, Bollerslev J, Khan AA, Shoback DM. Medical Management of Primary Hyperparathyroidism: Proceedings of the Fourth International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism. J Clin Endocrinol Metab. 2014 Oct 1;99(10):3607–18.
26. Khan A, Bilezikian J, Bone H, Gurevich A, Lakatos P, Misiorowski W, et al. Cinacalcet normalizes serum calcium in a double-blind randomized, placebo-controlled study in patients with primary hyperparathyroidism with contraindications to surgery. Eur J Endocrinol. 2015 May 1;172(5):527–35.
Kindly Let Us Know If This Was helpful? Thank You!


