
Polycystic ovary syndrome (PCOS) was eponymously named Stein-Leventhal syndrome based on a case series of seven women with clinical hyperandrogenism (hirsutism), oligomenorrhea, and enlarged ovaries, reported in 1935(1).
Interestingly, this condition may have been first described by Antonio Vallisneri, an Italian physician, in 1721. He described a young, obese, and infertile woman with enlarged ovaries like those of a dove’s eggs(2). In recent times, the definition of PCOS has undergone various modifications based on recommendations from different groups of experts(3–6).
These definitions, in part, depend on specific clinical, biochemical, or ultrasonographic features (see table 1). The pathophysiologic basis of PCOS is complex and is incompletely understood at this time(7). Optimal therapies for PCOS address various hormonal aberrations, which will be explored in this article.
Diagnostic criteria for PCOS
Table 1. Summary of diagnostic criteria for PCOS
| Diagnostic Feature | NIH (1990)(3) | ESHRE/ASRM(2003)(4) | AE-PCOS (2006)(5) | NIH extension of ESHRE/ASRM (2012)(6) |
| Hyperandrogenism (HA) | + | +/- | + | A, B, C |
| Oligo/amenorrhea(OA) | + | +/- | +/- | A, B, D |
| Polycystic Ovary morphology (PCOM) | +/- | +/- | A, C, D |
+ refers to a required diagnostic feature
+/- refers to a diagnostic feature that may or may not be present
NIH, National Institutes of Health (HA and OA required)
ESHRE/ASRM European Society for Human Reproduction and Embryology/American Society for Reproductive Medicine (also known as “Rotterdam Criteria”) (Any 2 of 3 diagnostic features)
AE-PCOS, Androgen Excess PCOS Society (HA with either OA or PCOM)
OD, Ovulatory Dysfunction (includes OA or other ovulatory dysfunction such as infertility)
A, Phenotype A (HA + OD + PCOM)
B, Phenotype B (HA + OD)
C, Phenotype C (HA + PCOM)
D, Phenotype D (OD + PCOM)
Phenotypes of PCOS
Polycystic ovary syndrome (PCOS) is a dysmetabolic and reproductive disorder associated with androgen excess in women.
- There are four separate phenotypes (A to D)
- Clinical or biochemical hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology (PCOm)
- Women affected by it are often overweight and have oligoanovulation and cutaneous manifestations of androgen excess (hirsutism, hyperseborrhea, acne, androgenetic alopecia).
Table 2. Summary of PCOS Phenotypes
| “Classic” PCOS (Phenotypes A and B) |
|
| “Ovulatory PCOS” (Phenotype C) |
|
| “Nonhyperandrogenic PCOS” (Phenotype D) |
|
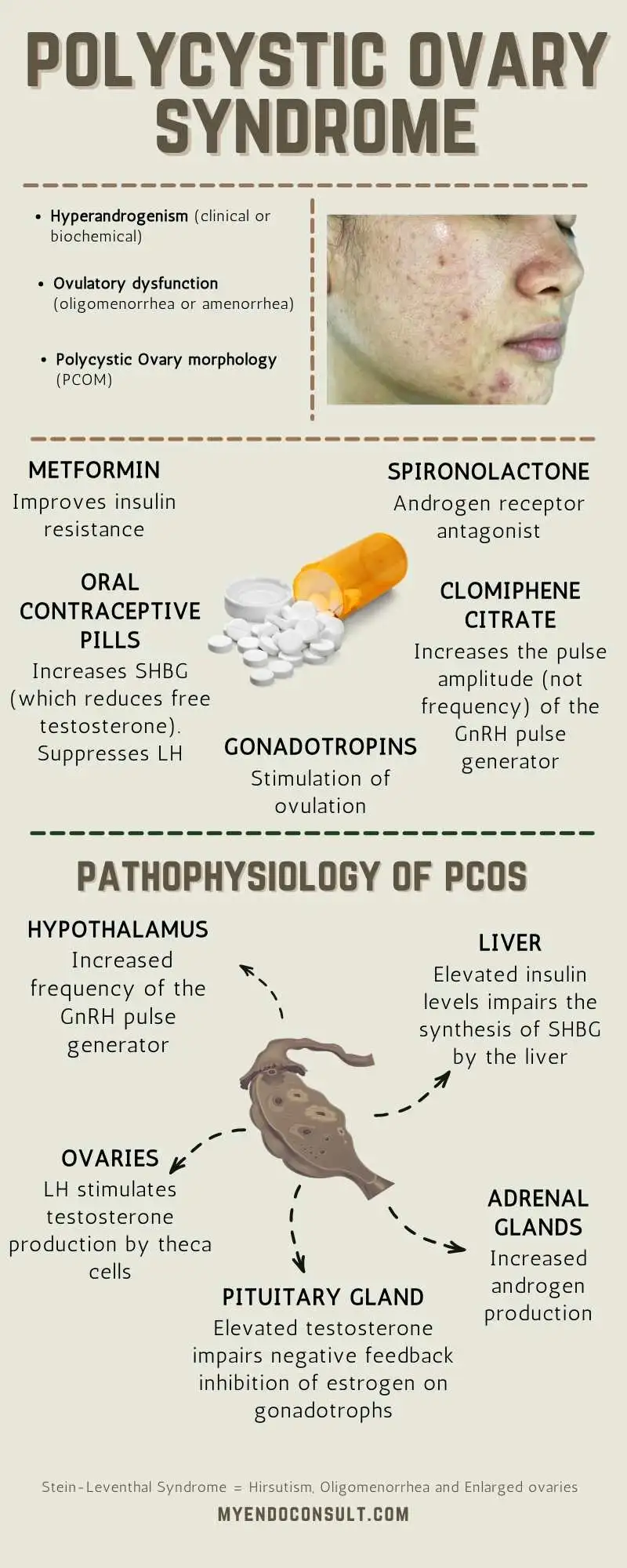
Pathophysiology of PCOS

Fig. 1 Pathophysiologic basis of PCOS. Data from references [33-38]
1) Increased activity of the GnRH pulse generator stimulates central gonadotrophs and results in an increase in luteinizing hormone (LH) with a concomitant decrease in follicle-stimulating hormone (FSH) (8,9).
2) LH stimulates theca cells of the ovaries to produce testosterone. Low FSH, on the other hand, results in less stimulation of the granulosa cell-mediated conversion of theca cell derived-testosterone into estrogens(10).
3) Reduced levels of circulating sex hormone-binding globulin (SHBG)(11) worsens the degree of hyperandrogenemia, as well. Both hyperandrogenemia and hyperinsulinemia impair hepatic SHBG synthesis(12), and since SHBG binds more avidly to estrogens than it does androgens, low levels of circulating SHBG increases the free androgen to free estrogen ratio.
4) Hyperandrogenemia impairs negative feedback effects of estrogen on the pituitary gonadotrophs, which results in unimpaired LH release and maintenance of a vicious cycle of hyperandrogenemia(13).
5) Adrenal-derived androgens play a contributory role, although the mechanisms are incompletely understood(14).
What are the reasons for insulin resistance in patients with PCOS?
- Post insulin to insulin-receptor binding defects contributes to insulin resistance in patients with PCOS.
- Reduction in glucose transporter 4 (GLUT-4) receptors in adipose tissue lead to reduced glucose uptake(15).
- Persistent hyperglycemia promotes rebound and persistent hyperinsulinemia. A prolonged period of hyperinsulinemia leads to progressive beta-cell dysfunction and death(15).
- Androgens may play a modest role in insulin resistance, although elevated serum androgens alone cannot explain insulin resistance in PCOS(15).
- Co-morbid dyslipidemia contributes to lipotoxicity-induced insulin resistance(16).
- There is also an increase, partially obesity-related, in inflammatory adipokines such as tumor-necrosis-factor-alpha (TNF-α), which induces insulin resistance. Furthermore, there is a concomitant decrease in the insulin-sensitizing adipokine, adiponectin(17,18).
Evaluation of PCOS
Common causes of secondary PCOS are hypothyroidism, Cushing’s syndrome, acromegaly, hyperprolactinemia, thyrotoxicosis, and late-onset CAH. These disorders are associated with androgen excess, variability in LH pulses, alterations in sex hormone-binding globulin (SHBG), and/or insulin resistance. Therefore, all patients with PCOS should have a baseline TSH, prolactin, and 17 α-hydroxyprogesterone to exclude secondary PCOS.
Clinical Features of PCOS (and their underlying pathophysiology)
Hirsutism
Hirsutism is defined as the presence of terminal, pigmented hair in a male-pattern distribution. It is a common dermatologic manifestation of polycystic ovary syndrome (PCOS) with a reported prevalence of 50-70%(19).
The original assessment of hirsutism using the Ferriman-Gallway score assigned a grade ranging from 0 to 4, depending on the presence and extent of terminal hair distribution involving 11 anatomic sites(20).
The forearm and lower leg have been excluded in the modified score since terminal hair growth in these areas are inconsistently associated with hyperandrogenemia. The modified Ferriman-Gallway score objectively assesses 9 anatomic regions for evidence of terminal hair growth(21). A modified Ferriman-Gallway score of ≥8 is consistent with clinically significant hirsutism(22,23).
Pathophysiology of hirsutism
Circulating androgens are responsible for hirsutism, although the degree of hirsutism is not necessarily determined by the severity of hyperandrogenemia. This has been attributed to interindividual differences in the response of hair follicles to androgens(19)
Hirsutism, like acne, and male pattern alopecia is dependent on the local abundance of 5-α-reductase (which reduces testosterone to active dihydrotestosterone) in the pilosebaceous unit(24).
The local concentration of 5-α-reductase in the skin varies by ethnicity. For example, hirsutism tends to be more common in women of Mediterranean background and less frequent and milder in women of East Asian or Native-American background(25–27).
Endocrine conditions associated with virilization
Adrenal tumors
Classic and non-classic congenital adrenal hyperplasia
Hyperandrogenism, insulin resistance, acanthosis nigricans (HAIR-AN syndrome)
Ovarian hyperthecosis
Ovarian tumors
The features of virilization include acne, male pattern baldness, deep male voice, and clitoral enlargement(28).
Acanthosis nigricans
Acanthosis nigricans (AN) is a classic dermatologic manifestation of PCOS and other endocrinopathies associated with insulin resistance. It is a velvety, dark, and plaque-like skin lesion which has a predilection for flexural areas such as the neck and axillary regions. 50% of patients with the classic obese PCOS phenotype have AN(29).
Pathophysiology of acanthosis nigricans
Hyperinsulinemia stimulates keratinocytes and fibroblasts directly, leading to their proliferation(29). Additional mechanisms accounting for hyperinsulinemia-induced hirsutism have been described earlier.
Acne
Acne is a common skin manifestation of PCOS, with a highly variable prevalence, based on ethnicity. Asian Indians have the highest prevalence, with the lowest being among Pacific Islanders(30).
Pathophysiology of acne
- Hyperinsulinemia in the setting of PCOS potentiates the excretion of sebum through the effects of insulin acting on IGF-1 receptors present on sweat glands(19). Accumulation of sebum establishes a milieu conducive for the proliferation of Propionibacterium acnes and eventual formation of comedones(31).
- Dihydrotesterone (an androgen) binds to its receptors on sweat glands and influences their output of sebum, as well(32). Interestingly, the severity of acne is not dependent on the degree of hyperandrogenemia and may be due to the variable sensitivity of pilosebaceous units to circulating androgens(33).
Obesity
The prevalence of obesity in patients with PCOS is between 40 to 80%. In contrast to women outside the United States(US), PCOS patients in the US have relatively higher body mass indexes (BMI’s)(34).
Pathophysiology of obesity
Circulating levels of androgen influence the distribution of body fat. Men have higher levels of testosterone, and as such, have a higher concentration of fat in the central abdomen compared to the hips or lower body.
Women with PCOS have high levels of testosterone, which changes the typical gynoid fat distribution into an android one. This is the reason for increased central or visceral adiposity(34).
More recent evidence refutes this hypothesis in women with PCOS. Although obese and non-obese women with PCOS had high levels of androgens compared to matched controls without PCOS, there was no difference in visceral adiposity between these two groups of PCOS subjects in this study(35).
The mechanisms remain incompletely understood at this time.
Mechanism of Action of PCOS therapies
Metformin
Metformin, a biguanide, is an insulin-sensitizing drug (ISD) that improves insulin resistance in PCOS. Although the mechanism of action of metformin has not been fully elucidated, it has been shown to activate the adenosine monophosphate-activated protein kinase (AMPK) pathway. A process that impairs hepatic glucose output reduces fatty acid oxidation and increases glucose uptake by peripheral tissues. See section 4.1.2 for additional information regarding the mechanism of action of metformin. By improving insulin resistance, metformin helps mitigate some hormonal perturbations in PCOS.
Practice Pearl(s)
- Metformin should not be used as a first-line pharmacological option in anovulatory infertility (PCOS).
- Metformin does not reduce the risk of spontaneous abortions in women with PCOS.
- For patients undergoing assisted reproductive techniques, concomitant metformin reduces the risk of ovarian hyperstimulation syndrome(36).
Clinical Trial Evidence
Table 3 Clinical trials assessing the role of metformin in PCOS.
| Population | Intervention | Outcome | |
| Hyperglycemia | Patients between 18-40 years of age with PCOS by the NIH criteria (n=39) | Metformin ER 1500mg per day for 12 weeks | IVGTT at baseline and study conclusion was compared. Insulin response to glucose increased (P =0.002), and basal glucose levels decreased (P=0.001) compared to baseline(37). |
| Hirsutism | Adult women with PCOS in 39 RCTs were analyzed in this Cochrane systematic review (n=2047) | Metformin compared to OCP | Metformin was less effective in improving hirsutism compared to OCP in patients with a BMI of 25-30kg/m2 ; (MD of 1.92, 95% CI 1.21-2.64)(38) |
| Menstrual regulation | Adult women with PCOS in 39 RCTs were analyzed in this Cochrane systematic review (n=2047) | Metformin compared to OCP | Metformin was less effective in improving menstrual patterns compared to OCP. (MD of 6.05, 95% CI 2.37-9.74)(38). |
| Infertility | This Cochrane systematic review analyzed adult women with PCOS in 6 RCTs. | ART in combination with metformin or placebo | Compared to placebo, Metformin treatment did not improve various reproductive endpoints, including pregnancies or live births. There was, however, a statistically significant reduction in the risk of OHSS (pooled OR of 0.27, 95% CI 0.16-0.47)) with metformin compared to placebo(39). |
ER extended-release; IVGTT intravenous glucose tolerance test
OCP oral contraceptive pill, BMI Body Mass Index, MD mean difference
ART assisted reproductive techniques
OR odds ratio
OHSS ovarian hyperstimulation syndrome
Oral contraceptive medications
Estrogen and progestin-containing oral contraceptive pills (OCPs) are pivotal in improving various clinical manifestations of PCOS, including oligomenorrhea, hirsutism, and acne.
Table 4. Sites of action of OCPs in PCOS
| Site of action | Estrogen | Progestin |
| GnRH pulse generator | Suppresses FSH > LH | Suppresses LH release |
| Androgens | Increases SHBG → Decreases free androgen | Decreases ovarian androgen production (impairs LH-mediated production of androgens by theca cells). Androgen receptor blocking effects‡. |
| Endometrium | Stabilizes the endometrium | Prevents endometrial hyperplasia. |
| Ovarian follicles | Suppresses recruitment and maturation of the dominant follicle | Inhibits the LH surge required for ovulation. |
‡ Androgen receptor blocking effects are due to specific progestins, e.g., drospirenone. Based on (40)
Therapeutic benefits of OCPs include regulating menstrual cycles, mitigating the clinical effects of hyperandrogenemia (such as hirsutism and acne), prevention of endometrial hyperplasia, and contraception (for patients on anti-androgen medications)(40).
Oral contraceptive pills with low androgenic or antiandrogenic progestin activity are reasonable in PCOS (e.g., drospirenone). However, it should be noted that no specific OCP has been shown in clinical trials to be the best option in PCOS; as such current practice guidelines do not recommend the use of a specific OCP in PCOS(41,42). Although progestin-only therapies (depot injections or oral) offer some endometrial protective benefits, they are associated with breakthrough (or prolonged) uterine bleeding(43).
Table 5. Progestogenic and androgenic potencies of low dose combined oral contraceptives
| Components of combined oral contraceptive | Activity of progestins | |||
| Brand name(s) | Estrogen(dose) | Progestin(dose) | Progestogenic | Androgenic |
| Orthonovum 1/35 | Ethinyl estradiol (35mcg) | Norethindrone(1mg) | +++ | +++ |
| Loestrin 1.5/30 | Ethinyl estradiol (30mcg) | Norethindrone(1.5mg) | ||
| Levlen, Nordette | Ethinyl estradiol (30mcg) | Levonorgestrel (0.15mg) | +++ | +++ |
| Levlite, Alesse | Ethinyl estradiol (20mcg) | Levonorgestrel (0.1mg) | ||
| Ortho Tri-Cyclen | Ethinyl estradiol (35mcg) | Norgestimate (0.18-0.25mg) | ++ | + |
| Ortho Tri-Cyclen LO | Ethinyl estradiol (25mcg) | Norgestimate (0.18-0.25mg) | ||
| Yasmin | Ethinyl estradiol (30mcg) | Drospirenone (3mg) | + | Antiandrogenic |
| Yaz | Ethinyl estradiol(20mcg) | Drospirenone (3mg) | ||
| Natazia | Estradiol valerate (1-3mg) | Dienogest(2-3mg) | + | Antiandrogenic |
Based on references (40,44)
Spironolactone (aldosterone antagonists)
Spironolactone, a diuretic and aldosterone antagonist, also has antagonistic properties at the androgen receptor(27). Additionally, spironolactone inhibits ovarian and adrenal steroidogenesis. Thus its utility in the management of acne and hirsutism in PCOS(45).
Additional antiandrogenic effects of spironolactone
- Inhibits the activity of 5 alpha-reductase (critical in converting testosterone to its active form, dihydrotestosterone)
- It increases the activity of aromatase (conversion of testosterone to estrogen)
- Increases sex hormone binding globulin
Practice Pearl(s)
Spironolactone, titrated to a dose of 200mg per day (for 6 to 9 months), is effective in improving moderate to severe hirsutism in patients with polycystic ovary syndrome(45,46). A starting dose of 25mg twice daily and increasing to a maximum dose of 100mg twice daily is reasonable.
Patients should be on oral contraceptive pills for at least six months before initiating spironolactone due to its effects on the development of male genitalia during intrauterine life. Furthermore, spironolactone can exacerbate menstrual irregularity if not used concomitantly with oral contraceptive therapy(27,47).
Spironolactone can cause a myriad of side effects due to its diuretic and anti-mineralocorticoid action. These include postural hypotension, dizziness, and hyperkalemia. The side effects are tolerable as long as patients on long-term therapy are closely monitored(48).
Clinical Trial Evidence
Table 6. Clinical trials assessing the role of spironolactone in PCOS
| Study | Population | Intervention | Outcome |
| Spironolactone plus metformin in PCOS | 56 overweight/obese patients with PCOS‡ | Randomized to metformin 1700mg daily (n=28) or metformin 1700mg + spironolactone 25mg daily (n=28) for 6 months | Modified Ferriman-Gallway scoreβ was significantly lower in the spironolactone + metformin group compared to the metformin only group ( p <0.001)(49) |
| Spironolactone versus metformin in PCOS (Open-label study) | 82 women with PCOS (NIH criteria) | Randomized to metformin 500mg twice a day (n= 41) or spironolactone 25mg twice a day (n=41). | Hirsutism score was significantly lower in the spironolactone group compared to the metformin group, with a much tolerable side effect profile(50) |
‡ Patients on oral contraceptives, anti-hypertensives, anti-diabetic or weight loss medications were excluded.
β Hirsutism score
Gonadotropins
The principal reason for infertility in PCOS is anovulation, making gonadotropins a suitable option for patients desiring fertility.
Table 7. Mechanism of action of gonadotropins in assisted reproduction.
| Agent | Mechanism(s) |
| Recombinant FSH (rFSH) | 1. FSH stimulates the recruitment and subsequent development of a cohort of pre-antral follicles(51,52). 2. FSH also rescues follicles destined for atresia(53). |
| Recombinant Luteinizing hormone (rLH), human chorionic gonadotropin hCG | 1. Enhances final follicular maturation. 2. Promotes maturation of the follicular oocyte from its germinal vesicle stage (prophase I of the cell cycle) to metaphase II of meiosis. 3. Maintain luteal function prior to the establishment of placental steroidogenesis(54). |
hCG binds to the LH receptor due to its structural homology to LH.
Practice Pearl(s)
Gonadotropin therapy is a suitable second-line option for patients with PCOS who are clomiphene or letrozole resistant.
Table 8. Dosing schedule of gonadotropins
| Treatment | Dose and timing |
| rFSH‡ | 75 IU administered subcutaneously on a daily basis for to up 14 days until follicular maturity is achieved (55). |
| hCG∆ | 5,000-10,000 IU administered intramuscularly or subcutaneously. |
‡Dose of rFSH is increased on a weekly basis if there is a suboptimal response. Treatment is terminated after 35 days if there is a suboptimal response(55)—monitoring of response through transvaginal ultrasounds and serial serum estradiol levels. If more than four follicles have a diameter of 14mm or greater, the cycle is canceled due to the risk of ovarian hyperstimulation syndrome(56).
∆ Progesterone concentration above 5ng/ml confirms ovulation. Follicular rupture occurs within 48hours of hCG administration(57). (Progesterone levels assessed on day 7 and pregnancy test on day 14).
Clinical Trial Evidence
This systematic review measured various outcomes among women with PCOS treated with gonadotropins (urinary gonadotropins were compared to rFSH). All study participants had previously failed clomiphene citrate therapy. Those on co-treatment with metformin, letrozole, LH, or clomiphene citrate were excluded from the systematic review.
Table 9. Comparison of fertility outcomes of gonadotropin preparations
| Study endpoint | Urinary-derived gonadotropins | Recombinant FSH | OR (95% CI) |
| Live birth rate per woman | 157 per 1000 | 191 per 1000 | 1.26 (0.80-1.99) |
| Incidence of OHSS | 22 per 1000 | 33 per 1000 | 1.52 (0.81-2.84) |
| Incidence of multi pregnancy rate per woman | 30 per 1000 | 26 per 1000 | 0.86 (0.44-1.65) |
OHSS ovarian hyperstimulation syndrome
OR odds ratio
Urinary-derived gonadotropins included FSH, human menopausal gonadotropin (HMG), purified FSH, highly purified FSH, and highly purified HMG.
There was no statistically significant difference between comparisons of gonadotropins in terms of live birth rate, the incidence of multigestational pregnancies, or OHSS(58).
Clomiphene Citrate
Clomiphene demonstrates its therapeutic effects in the setting of an intact hypothalamic-pituitary axis and optimal endogenous estrogen production (e.g., PCOS). Clomiphene is a selective estrogen receptor modular (SERM); as such, it can bind to estrogen receptors in various sensitive tissues, including the hypothalamus, ovaries, and endometrial lining(59). Furthermore, it can exert both partial agonist and antagonist effects depending on the tissue in question.
For example, at the level of the hypothalamus, clomiphene acts as an antagonist, thus preventing negative feedback inhibition of the GnRH pulse generator by endogenous estrogen(60). In patients with PCOS, clomiphene increases the pulse amplitude but not the pulse frequency of the GnRH pulse generator. As a result, the levels of LH and FSH increases, thereby enhancing the recruitment and maturation of ovarian follicles(61).
Practice Pearl(s)
Clomiphene citrate is traditionally initiated within 5 days of spontaneous menstruation or progestin-induced withdrawal bleeding. The starting dose of clomiphene citrate is 50mg daily for 5 days. Ovulation is expected within 5 to 10 days after the last dose of clomiphene citrate; therefore, ovulation monitoring is required within this time window. If there is therapeutic failure, the dose of clomiphene citrate can be increased by 50mg/day during the next cycle, up to a maximum daily dose of 250mg(59).
Alternative indications for clomiphene use
- Male hypogonadism (62)
- Gynecomastia (it is, however, not as effective as clomiphene)(63)
Clinical Trial Evidence
In this meta-analysis of 3 trials and 133 participants with PCOS, clomiphene citrate resulted in a statistically significant increase in pregnancy and ovulation rates per woman, compared to placebo(64).
Table 10. A meta-analysis of clomiphene citrate versus placebo in PCOS
| Study endpoint | Clomiphene Citrate | Placebo | OR (95% CI) |
| Pregnancy rate (per woman) | 14 | 2 | 5.77 (1.55-21.48) |
| Ovulation rate (per woman) | 45 | 14 | 7.47 (3.24-17.23) |
OR odds ratio
Letrozole
Letrozole is a competitive nonsteroidal aromatase inhibitor that exerts its therapeutic effects in PCOS by blocking the conversion of androgens to estrogens. Low circulating estrogen levels blunts its negative feedback effect on the GnRH pulse generator, which subsequently increases GnRH pulse frequency and gonadotropin release(65).
Practice Pearl(s)
Letrozole at a dose of 2.5mg daily is initiated after three days of either spontaneous menstruation or progestin-induced bleed (medroxyprogesterone acetate, 5mg daily for 10 days). Like clomiphene, letrozole is administered for 5 consecutive days. Furthermore, for patients who experience a poor ovulatory response, a gradual increase in dose to 7.5mg daily during subsequent cycles is recommended, for a total of 5 cycles(66).
Clinical Trial Evidence
In this meta-analysis of patients with PCOS, letrozole was compared to clomiphene citrate. Letrozole resulted in a statistically significant increase in clinical pregnancy rates and live birth rates compared to clomiphene. There was no statistically significant difference in ovarian hyperstimulation syndrome rates, miscarriage rates, or multigestational rates(67).
Table 11. A meta-analysis of clomiphene citrate versus letrozole in PCOS
| Study endpoint | Clomiphene Citrate | Letrozole | OR (95% CI) |
| Live birth rate | 214 per 1000 | 314 per 1000 | 1.68 (1.42-1.99) |
| Clinical pregnancy rate | 264 per 1000 | 359 per 1000 | 1.56 (1.37-1.78) |
OR odds ratio
Androgen receptor antagonist
Table 12. Mechanism of action of androgen receptor blockers
| Drug | Mechanism of Action | Clinical Effects |
| Spironolactone | Aldosterone receptor blocker that inhibits the binding of testosterone and dihydrotestosterone to the androgen receptor. Increases SHBG and inhibits 5 alpha-reductase | Treatment of acne and alopecia, hirsutism |
| Flutamide | A selective non-steroidal androgen receptor blocker. | Treatment of acne, hirsutism, and alopecia. |
Based on reference (32)
Practice Pearl(s)
Table 13. Androgen receptor blockers in clinical practice
| Drug | Dosage | Monitoring/Side effects |
| Spironolactone | 100-200 mg per day in two divided doses. | Female patients should be on concomitant contraceptive therapy. It can cause gastrointestinal discomfort and menstrual irregularity(68). |
| Flutamide | 62.5-250 mg per day | Combination therapy with oral contraceptive therapy is recommended(69). It is associated with hepatotoxicity (close monitoring of liver function tests is required)(70). |
Based on references (68–70)
Clinical Trial Evidence
In this six-month randomized study, 48 patients with hirsutism were randomized to either 100mg per day of cyproterone acetate or 100mg per day of spironolactone. All subjects received additional therapy with estrogen therapy (contraception). Compared to baseline, both cyproterone acetate and spironolactone led to a 16.8% (p-value < 0.001) and 17.1% ( p-value < 0.001) decline in total hair diameter, respectively. More importantly, there was no between-group difference in their effect of total hair diameter(71).
Alpha-reductase inhibitor
Finasteride impairs the conversion of testosterone to dihydrotestosterone (the active metabolite of testosterone) by inhibiting the type 2 isoenzyme of 5 alpha-reductase(72). §
Practice Pearl(s)
It is potentially teratogenic and is prescribed off-label in androgenetic alopecia and hirsutism. The recommended dose range of oral finasteride is 0.5 to 5mg per day(73).
Finasteride gel 0.25%, applied twice daily is efficacious in treating both hirsutism and acne, with very minimal side effects(74).
Clinical Trial Evidence
In this controlled clinical study by Tolino et al, 15 women with polycystic ovary syndrome were treated with oral finasteride 5mg daily for a period of 6 months. The mean Ferriman-Gallway score (clinical assessment of the degree of hirsutism) declined from 24.6 (with a standard deviation of 1.8) at baseline, to 8.9 (standard deviation of 2.4) after six months of therapy (P value of <0.05)(75).
References
- Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935 Jan 1;29(2):181–91.
- Battaglia C. The role of ultrasound and Doppler analysis in the diagnosis of polycystic ovary syndrome. Ultrasound Obstet Gynecol. 2003;22(3):225–32.
- Zawadzski J. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. Polycystic Ovary Syndr. 1992;39–50.
- The Rotterdam ESHRE/ASRM‐sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004 Jan 1;19(1):41–7.
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Criteria for Defining Polycystic Ovary Syndrome as a Predominantly Hyperandrogenic Syndrome: An Androgen Excess Society Guideline. J Clin Endocrinol Metab. 2006 Nov 1;91(11):4237–45.
- Johnson T, Kaplan L, Ouyang P, Rizza P. National Institutes of Health Evidence-Based Methodology Workshop on Polycystic Ovary Syndrome. NIH EbMW Reports. Bethesda, MD: National Institutes of Health, 2012; 1: 1–14. Exec Summ Available Httpsprevention Nih GovdocsprogramspcosFinalReport Pdf. 2019;1–14.
- Ibáñez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E, et al. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Horm Res Paediatr. 2017;88:371–95.
- Leondires MP, Berga SL. Role of GnRH drive in the pathophysiology of polycystic ovary syndrome. J Endocrinol Invest. 1998 Aug;21(7):476–85.
- Chaudhari N, Dawalbhakta M, Nampoothiri L. GnRH dysregulation in polycystic ovarian syndrome (PCOS) is a manifestation of an altered neurotransmitter profile. Reprod Biol Endocrinol. 2018 Apr 11;16(1):37.
- Johansson J, Stener-Victorin E. Polycystic Ovary Syndrome: Effect and Mechanisms of Acupuncture for Ovulation Induction. Evid-Based Complement Altern Med ECAM [Internet]. 2013 [cited 2019 Jan 4];2013. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3773899/
- Deswal R, Yadav A, Dang AS. Sex hormone binding globulin – an important biomarker for predicting PCOS risk: A systematic review and meta-analysis. Syst Biol Reprod Med. 2018 Feb;64(1):12–24.
- Mehrabian F, Afghahi M. Can Sex-hormone Binding Globulin Considered as a Predictor of Response to Pharmacological Treatment in Women with Polycystic Ovary Syndrome? Int J Prev Med. 2013 Oct;4(10):1169–74.
- Bremer AA. Polycystic ovary syndrome in the pediatric population. Metab Syndr Relat Disord. 2010 Oct;8(5):375–94.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016 Nov;37:80–97.
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997 Dec;18(6):774–800.
- Yazıcı D, Sezer H. Insulin Resistance, Obesity and Lipotoxicity. Adv Exp Med Biol. 2017;960:277–304.
- Chen X, Jia X, Qiao J, Guan Y, Kang J. Adipokines in reproductive function: a link between obesity and polycystic ovary syndrome. J Mol Endocrinol. 2013 Apr;50(2):R21-37.
- Dimitriadis GK, Kyrou I, Randeva HS. Polycystic Ovary Syndrome as a Proinflammatory State: The Role of Adipokines. Curr Pharm Des. 2016;22(36):5535–46.
- Feng JG, Guo Y, Ma LA, Xing J, Sun RF, Zhu W. Prevalence of dermatologic manifestations and metabolic biomarkers in women with polycystic ovary syndrome in north China. J Cosmet Dermatol. 2018 Jun;17(3):511–7.
- Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961 Nov;21:1440–7.
- Cook H, Brennan K, Azziz R. Reanalyzing the modified ferriman-gallwey score: is there a simpler method for assessing the extent of hirsutism? Fertil Steril. 2011 Nov;96(5):1266-1270.e1.
- Aswini R, Jayapalan S. Modified Ferriman–Gallwey Score in Hirsutism and its Association with Metabolic Syndrome. Int J Trichology. 2017;9(1):7–13.
- Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981 Aug 1;140(7):815–30.
- Rosenfield RL. Clinical practice. Hirsutism. N Engl J Med. 2005 Dec 15;353(24):2578–88.
- Coskun A, Ercan O, Arikan DC, Özer A, Kilinc M, Kiran G, et al. Modified Ferriman–Gallwey hirsutism score and androgen levels in Turkish women. Eur J Obstet Gynecol Reprod Biol. 2011 Feb 1;154(2):167–71.
- Escobar-Morreale HF, Carmina E, Dewailly D, Gambineri A, Kelestimur F, Moghetti P, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012 Mar 1;18(2):146–70.
- Martin KA, Anderson RR, Chang RJ, Ehrmann DA, Lobo RA, Murad MH, et al. Evaluation and Treatment of Hirsutism in Premenopausal Women: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018 Apr 1;103(4):1233–57.
- Mihailidis J, Dermesropian R, Taxel P, Luthra P, Grant-Kels JM. Endocrine evaluation of hirsutism. Int J Womens Dermatol. 2017 Feb 16;3(1 Suppl):S6–10.
- Panidis D, Skiadopoulos S, Rousso D, Ioannides D, Panidou E. Association of acanthosis nigricans with insulin resistance in patients with polycystic ovary syndrome. Br J Dermatol. 1995 Jun;132(6):936–41.
- Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005 Aug;90(8):4650–8.
- Chuan SS, Chang RJ. Polycystic ovary syndrome and acne. Skin Ther Lett. 2010 Dec;15(10):1–4.
- Ju Q, Tao T, Hu T, Karadağ AS, Al-Khuzaei S, Chen W. Sex hormones and acne. Clin Dermatol. 2017 Apr;35(2):130–7.
- Khezrian L, Yazdanfar A, Azizian Z, Hassani P, Feyzian M. The Relationship Between Acne and Other Hyperandrogenism Signs. J Skin Stem Cell. 2016;3(3):e64187.
- Sam S. Obesity and Polycystic Ovary Syndrome. Obes Manag. 2007 Apr;3(2):69–73.
- Boumosleh JM, Grundy SM, Phan J, Neeland IJ, Chang A, Vega GL. Metabolic Concomitants of Obese and Nonobese Women With Features of Polycystic Ovarian Syndrome. J Endocr Soc. 2017 Nov 2;1(12):1417–27.
- Hashim HA. Twenty years of ovulation induction with metformin for PCOS; what is the best available evidence? Reprod Biomed Online. 2016 Jan 1;32(1):44–53.
- Pau CT, Keefe C, Duran J, Welt CK. Metformin Improves Glucose Effectiveness, Not Insulin Sensitivity: Predicting Treatment Response in Women With Polycystic Ovary Syndrome in an Open-Label, Interventional Study. J Clin Endocrinol Metab. 2014 May;99(5):1870–8.
- Fraison E, Kostova E, Moran LJ, Bilal S, Ee CC, Venetis C, et al. Metformin versus the combined oral contraceptive pill for hirsutism, acne, and menstrual pattern in polycystic ovary syndrome. Cochrane Database Syst Rev. 2020 Aug 13;2020(8):CD005552.
- Tso LO, Costello MF, Albuquerque LE, Andriolo RB, Freitas V. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2009 Apr 15;(2):CD006105.
- Nader S, Diamanti-Kandarakis E. Polycystic ovary syndrome, oral contraceptives and metabolic issues: new perspectives and a unifying hypothesis. Hum Reprod. 2007 Feb 1;22(2):317–22.
- Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and Treatment of Polycystic Ovary Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2013 Dec 1;98(12):4565–92.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 194: Polycystic Ovary Syndrome. Obstet Gynecol. 2018 Jun;131(6):e157–71.
- Kovacs G. Progestogen-only pills and bleeding disturbances. Hum Reprod Oxf Engl. 1996 Oct;11 Suppl 2:20–3.
- Shah D, Patil M. Consensus Statement on the Use of Oral Contraceptive Pills in Polycystic Ovarian Syndrome Women in India. J Hum Reprod Sci. 2018;11(2):96–118.
- Armanini D, Andrisani A, Bordin L, Sabbadin C. Spironolactone in the treatment of polycystic ovary syndrome. Expert Opin Pharmacother. 2016 Sep 1;17(13):1713–5.
- Cumming DC, Yang JC, Rebar RW, Yen SS. Treatment of hirsutism with spironolactone. JAMA. 1982 Mar 5;247(9):1295–8.
- Spritzer PM, Lisboa KO, Mattiello S, Lhullier F. Spironolactone as a single agent for long-term therapy of hirsute patients. Clin Endocrinol (Oxf). 2000;52(5):587–94.
- Williams EM, Katholi RE, Karambelas MR. Use and side-effect profile of spironolactone in a private cardiologist’s practice. Clin Cardiol. 2006 Apr;29(4):149–53.
- Mazza A, Fruci B, Guzzi P, D’Orrico B, Malaguarnera R, Veltri P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovasc Dis NMCD. 2014 Feb;24(2):132–9.
- Ganie MA, Khurana ML, Eunice M, Gulati M, Dwivedi SN, Ammini AC. Comparison of Efficacy of Spironolactone with Metformin in the Management of Polycystic Ovary Syndrome: An Open-Labeled Study. J Clin Endocrinol Metab. 2004 Jun 1;89(6):2756–62.
- Macklon NS, Fauser BC. Follicle-stimulating hormone and advanced follicle development in the human. Arch Med Res. 2001 Dec;32(6):595–600.
- Kovacs P, Sajgo A, Kaali SG, Pal L. Detrimental effects of high-dose gonadotropin on outcome of IVF: making a case for gentle ovarian stimulation strategies. Reprod Sci Thousand Oaks Calif. 2012 Jul;19(7):718–24.
- Hsueh AJW, Kawamura K, Cheng Y, Fauser BCJM. Intraovarian Control of Early Folliculogenesis. Endocr Rev. 2015 Feb 2;36(1):1–24.
- Leão R de BF, Esteves SC. Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech. Clinics. 2014 Apr;69(4):279–93.
- Homburg R, Howles CM. Low-dose FSH therapy for anovulatory infertility associated with polycystic ovary syndrome: rationale, results, reflections and refinements. Hum Reprod Update. 1999 Oct;5(5):493–9.
- Streda R, Mardesic T, Sobotka V, Koryntova D, Hybnerova L, Jindra M. Comparison of different starting gonadotropin doses (50, 75 and 100 IU daily) for ovulation induction combined with intrauterine insemination. Arch Gynecol Obstet. 2012;286(4):1055–9.
- Fischer RA, Nakajima ST, Gibson M, Brumsted JR. Ovulation after intravenous and intramuscular human chorionic gonadotropin. Fertil Steril. 1993 Sep;60(3):418–22.
- Ns W, M N, N B, Bw M, F V der V, M van W. Gonadotrophins for ovulation induction in women with polycystic ovarian syndrome. Cochrane Database Syst Rev [Internet]. 2015 Sep 9 [cited 2022 Jan 30];(9). Available from: https://pubmed.ncbi.nlm.nih.gov/26350625/
- Von Hofe J, Bates GW. Ovulation induction. Obstet Gynecol Clin North Am. 2015 Mar;42(1):27–37.
- Adashi EY. Clomiphene citrate: mechanism(s) and site(s) of action–a hypothesis revisited. Fertil Steril. 1984 Sep;42(3):331–44.
- Kettel LM, Roseff SJ, Berga SL, Mortola JF, Yen SS. Hypothalamic-pituitary-ovarian response to clomiphene citrate in women with polycystic ovary syndrome. Fertil Steril. 1993 Mar;59(3):532–8.
- Bach PV, Najari BB, Kashanian JA. Adjunct Management of Male Hypogonadism. Curr Sex Health Rep. 2016 Dec 1;8(4):231–9.
- Agrawal S, Ganie MA, Nisar S. Gynaecomastia. In: Kumar A, Sharma M, editors. Basics of Human Andrology: A Textbook [Internet]. Singapore: Springer; 2017 [cited 2022 Jan 30]. p. 451–8. Available from: https://doi.org/10.1007/978-981-10-3695-8_26
- Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Syst Rev. 2009 Oct 7;(4):CD002249.
- Casper RF, Mitwally MFM. Use of the aromatase inhibitor letrozole for ovulation induction in women with polycystic ovarian syndrome. Clin Obstet Gynecol. 2011 Dec;54(4):685–95.
- Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014 Jul 10;371(2):119–29.
- Franik S, Eltrop SM, Kremer JA, Kiesel L, Farquhar C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2018 May 24;5:CD010287.
- Cumming DC. Use of spironolactone in treatment of hirsutism. Cleve Clin J Med. 1990 May;57(3):285–7.
- Generali JA, Cada DJ. Flutamide: Hirsutism in Women. Hosp Pharm. 2014 Jun;49(6):517.
- Iguchi T, Tamada S, Kato M, Yasuda S, Otoshi T, Hamada K, et al. Enzalutamide versus flutamide for castration-resistant prostate cancer after combined androgen blockade therapy with bicalutamide: a retrospective study. Int J Clin Oncol. 2019 Jul;24(7):848–56.
- O’Brien RC, Cooper ME, Murray RM, Seeman E, Thomas AK, Jerums G. Comparison of sequential cyproterone acetate/estrogen versus spironolactone/oral contraceptive in the treatment of hirsutism. J Clin Endocrinol Metab. 1991 May;72(5):1008–13.
- Moghetti P, Tosi F, Tosti A, Negri C, Misciali C, Perrone F, et al. Comparison of Spironolactone, Flutamide, and Finasteride Efficacy in the Treatment of Hirsutism: A Randomized, Double Blind, Placebo-Controlled Trial1. J Clin Endocrinol Metab. 2000 Jan 1;85(1):89–94.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019 Jul;58(7):759–76.
- Tahvilian R, Ebrahimi A, Beiki O, Nemati H, Masoud S. Preparation and clinical evaluation of Finastride gel in the treatment of idiopathic Hirsutism. J Drug Assess. 2015;4(1):12.
- Tolino A, Petrone A, Sarnacchiaro F, Cirillo D, Ronsini S, Lombardi G, et al. Finasteride in the treatment of hirsutism: new therapeutic perspectives. Fertil Steril. 1996 Jul;66(1):61–5.
Explore the pathophysiology of various endocrine diseases and the mechanism of action of medications utilized in their treatment. Click here to learn more!
Kindly Let Us Know If This Was helpful? Thank You!


