Understanding Hirsutism
Hirsutism, a condition characterized by excessive terminal hair appearing in a male pattern on women, predominantly occurs in androgen-dependent areas. The majority of hirsutism cases, approximately 80% or more, are due to androgen excess.
About 70%-80% of women suffering from hirsutism are also diagnosed with Polycystic Ovary Syndrome (PCOS), making it the most common underlying cause. Idiopathic hirsutism, with no identifiable cause, constitutes 5% to 20% of hirsute women.
Differential Diagnosis of Hirsutism
Various conditions can cause hirsutism, including:
- PCOS
- Non-classical adrenal hyperplasia (NCCAH)
- Androgen-secreting tumors, which are present in 0.2% of hyperandrogenic women and more than half are malignant
- Ovarian hyperthecosis
- Cushing’s syndrome
- Acromegaly
- Hypothyroidism
- Hyperprolactinemia
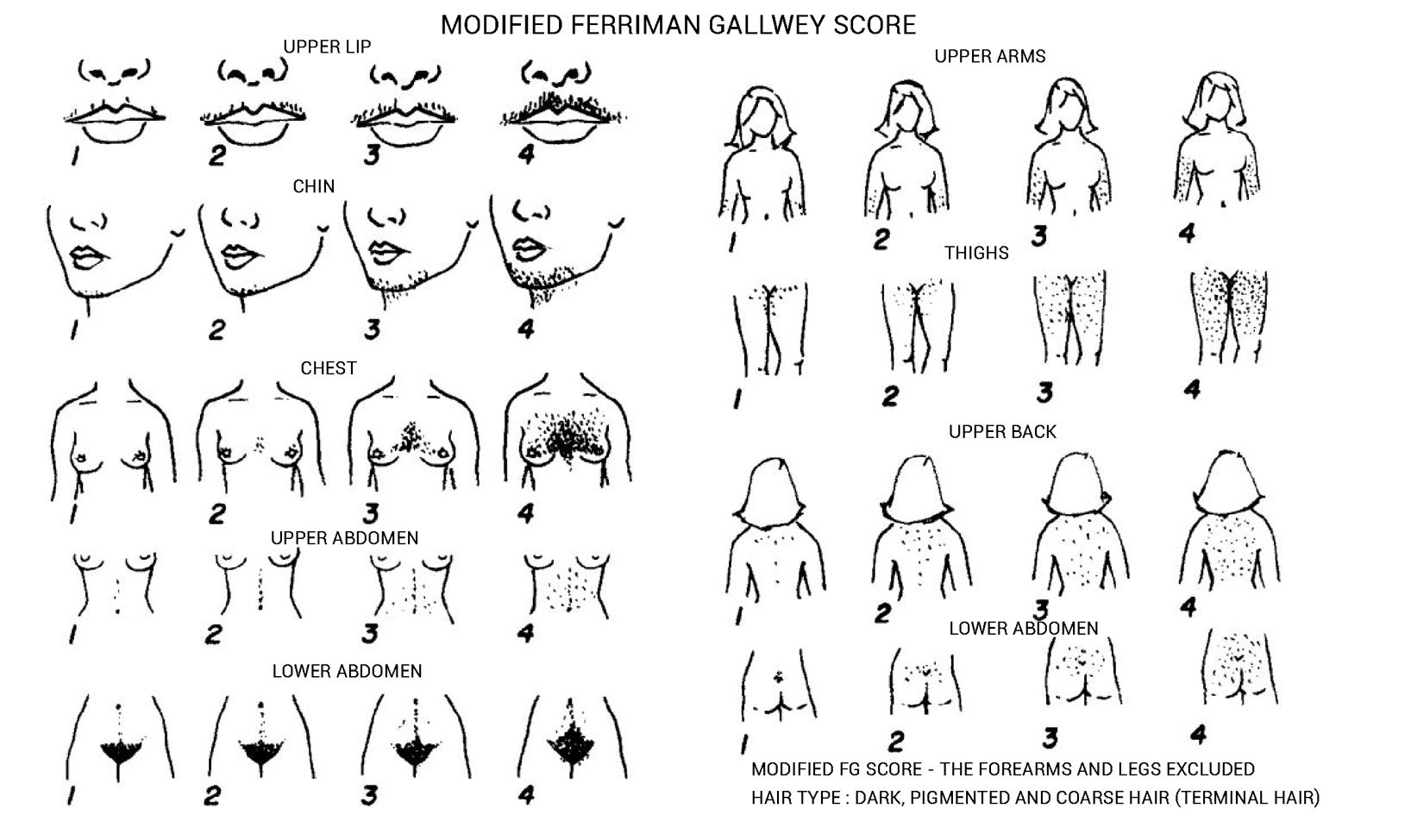
Ferriman-Gallwey Hirsutism Scoring System
The Ferriman-Gallwey Hirsutism scoring system assigns a score from 0 (no hair) to 4 (frankly virile) to each of the nine body areas most sensitive to androgen. The scores from each area are summed to provide a total hormonal hirsutism score. Generalized hirsutism (score ≥8) is considered abnormal in the general U.S. population, while locally excessive hair growth (score <8) is a common normal variant.
The hirsutism score differs across various populations:
- For black or white women in the United States and the United Kingdom, a score of ≥8 is considered hirsutism.
- Mediterranean, Hispanic, and Middle Eastern women are considered hirsute at scores of ≥9 to 10.
- South American women: ≥6
- Among Asian women, a score of ≥2 is considered hirsutism for Han Chinese women, while for Southern Chinese women the score is ≥7.
Non-classical Adrenal Hyperplasia (NCCAH)
NCCAH is caused by a late-onset deficiency of the CYP21A2 enzyme and is present in 4.2% of hyperandrogenic women worldwide. In whites, the prevalence of the nonclassic form can be as high as 1 in 1000 to 1 in 100, and even higher among Mediterraneans, Hispanics, and Eastern European Jews. NCCAH is an autosomal recessive condition that can present during late childhood with symptoms such as premature pubarche, acne, and accelerated bone age, or in adolescent and adult females with acne, hirsutism, and menstrual irregularity. Women with the late-onset form may be either compound heterozygotes (with a classic mutation and a variant allele) or heterozygotes with two variant alleles, resulting in 20 to 60% of normal enzymatic activity.
Initial Screening
The first step in diagnosing NCCAH involves an initial screening. This requires a morning (7:30 to 8 AM) serum sample for 17-hydroxyprogesterone concentration, which is obtained during the follicular phase of the menstrual cycle for women with regular cycles. For women with amenorrhea or infrequent menses, the sample can be taken on a random day.
Confirming NCCAH
If the 17-hydroxyprogesterone concentration in the basal sample is >200 ng/dL, the next step involves an Adrenocorticotropic Hormone (ACTH) stimulation test. A serum 17-hydroxyprogesterone value exceeding 1000 ng/dL on this test confirms the diagnosis of NCCAH.
Ovarian Androgen-Secreting Tumors
These types of tumors include Sertoli-Leydig cell tumors (androblastoma, arrhenoblastoma), Granulosa-theca cell (stromal cell) tumors, and Hilus cell tumors. Such conditions often present with serum testosterone concentrations greater than 150 to 200 ng/dL. Hirsutism usually appears later in life and progresses rapidly, often leading to frank virilization characterized by clitoromegaly and a deepening of the voice.
Adrenal Androgen-Secreting Tumors
Adrenal tumors, though rare, can cause androgen excess. These tumors secrete testosterone, androgens (DHEA and DHEAS), and cortisol. These conditions can manifest as clinical symptoms of androgen excess and Cushing’s syndrome. It’s worth noting that some carcinomas may lose the ability to sulfate DHEA, so a normal serum DHEAS value does not exclude the diagnosis.
Ovarian Hyperthecosis
Ovarian hyperthecosis is a nonmalignant ovarian disorder that leads to increased production of testosterone by luteinized thecal cells in the stroma, resulting in increased serum testosterone concentrations. It is unclear if hyperthecosis is a distinct disorder or part of the spectrum of PCOS. This condition typically manifests as gradual onset of hirsutism and frank virilization, and is primarily observed in postmenopausal women.
Addressing Hirsutism: Evaluation
Initial Lab Tests
The initial lab tests recommended to help differentiate between these conditions include:
- Total testosterone +/- free testosterone
- 17-OH progesterone
- Prolactin
- Thyroid-stimulating hormone (TSH)
Additional Testing
In some cases, additional testing might be required. These tests could include:
- Luteinizing Hormone (LH)
- Follicle-Stimulating Hormone (FSH)
- Dehydroepiandrosterone Sulfate (DHEA-S)
- Screening for Cushing’s syndrome, which includes a Dexamethasone suppression test
- Pelvic or transvaginal ultrasound
- CT scan of the abdomen and pelvis
Treatment of Hirsutism
The treatment of hirsutism often depends on its severity and the patient’s medical history.
- For Most Women with Patient-Important Hirsutism
Pharmacological therapy is often the first-line treatment. This might include oral contraceptives or antiandrogens, which are typically initiated if hirsutism persists after six months of treatment with oral contraceptives. Antiandrogen medications used may include spironolactone (100-200 mg/day in divided doses) and finasteride (2.5-5 mg/day). Direct hair removal methods like photoepilation and electrolysis may also be used.
- In Patients with Severe Hirsutism Causing Emotional Distress
For women who have not experienced sufficient improvement with oral contraceptives, it may be necessary to initiate combination therapy with an oral contraceptive and an antiandrogen.
- For Women with Mild Hirsutism and No Evidence of an Endocrine Disorder
For these patients, pharmacologic therapy or direct hair removal methods can be used as initial therapy.
Despite their effectiveness, antiandrogens are not usually recommended as initial therapy due to their potential to cause birth defects. However, they can be used as initial therapy in women who are not sexually active, women who have undergone permanent sterilization, or women who are using long-acting reversible contraception.
Identifying and Managing Complications in Women with PCOS
While managing hirsutism is a crucial part of PCOS treatment, it is also essential to screen for and address various complications associated with this condition.

Screening for Complications
The complications associated with PCOS require vigilant screening and may include the following:
- Obesity: This is evaluated through BMI calculation and measurement of waist circumference.
- Cardiovascular Risk: Assessment is crucial, and statin therapy may be considered if indicated.
- Sleep-Disordered Breathing/Obstructive Sleep Apnea (OSA)
- Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH)
- Depression and Anxiety
- Endometrial Cancer: Screening is recommended, particularly through transvaginal ultrasound to assess endometrial thickness in cases of unexpected uterine bleeding or spotting.
- Type 2 Diabetes Mellitus: Screening is advised through an oral glucose tolerance test (OGTT), or a HgbA1c test if the patient cannot or is unwilling to complete an OGTT. Rescreening is recommended every 3–5 years, or more frequently if clinical factors or symptoms of diabetes develop.

Metformin and PCOS
Metformin is recommended for women with PCOS who have Type 2 Diabetes Mellitus (T2DM) or Impaired Glucose Tolerance (IGT) and fail lifestyle modification. Additionally, it can be used as second-line therapy for women with PCOS and menstrual irregularity who cannot take or do not tolerate hormonal contraceptives (HCs).
GLP-1 Receptor Agonists and PCOS
GLP-1 receptor agonists have been found to be effective for weight loss and reducing waist circumference. They may also help with menstrual irregularity, ovulation, fertility (though data is limited), and a reduction in cardiovascular risk markers. However, these drugs should be discontinued two months prior to conception due to limited data on their use during pregnancy.
GLP-1/GIP Receptor Agonist: Tirzepatide
Although further data is needed on its effects in women with PCOS, females using oral contraceptives are advised to switch to a non-oral contraceptive method, or add a barrier method of contraception for four weeks after initiation and for four weeks after each dose escalation. The use of tirzepatide during pregnancy is not recommended due to limited data.
Treatment of Infertility
In cases of anovulatory infertility in women with PCOS, clomiphene citrate or letrozole is often recommended as the first-line treatment. The Reproductive Network Trial showed a greater number of live births with letrozole, particularly in obese women with PCOS.
Metformin can be used as an adjuvant therapy for infertility to prevent ovarian hyperstimulation syndrome (OHSS) in women with PCOS undergoing in vitro fertilization (IVF). When used to induce ovulation in PCOS, it should be discontinued by the end of the first trimester.
Conclusion
In summary, hirsutism, as seen particularly in women with PCOS and NCCAH, is a condition requiring careful evaluation and differential diagnosis. The etiological factors range from androgen-secreting tumors to various endocrine disorders like Cushing’s syndrome, hypothyroidism, and hyperprolactinemia, underscoring the importance of comprehensive assessment and personalized treatment approaches.
The management of hirsutism includes both pharmacological interventions such as oral contraceptives, antiandrogens, and metformin, as well as mechanical hair removal techniques. Additional attention should be given to associated complications, including obesity, cardiovascular disease, type 2 diabetes, sleep disorders, and endometrial cancer, among others, as part of the overall treatment strategy.
References
Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, Christman GM, Huang H, Yan Q, Alvero R, Haisenleder DJ, Barnhart KT, Bates GW, Usadi R, Lucidi S, Baker V, Trussell JC, Krawetz SA, Snyder P, Ohl D, Santoro N, Eisenberg E, Zhang H; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014 Jul 10;371(2):119-29. doi: 10.1056/NEJMoa1313517. Erratum in: N Engl J Med. 2014 Oct 9;317(15):1465. PMID: 25006718; PMCID: PMC4175743.
Martin KA, Chang RJ, Ehrmann DA, Ibanez L, Lobo RA, Rosenfield RL, Shapiro J, Montori VM, Swiglo BA. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008 Apr;93(4):1105-20. doi: 10.1210/jc.2007-2437. Epub 2008 Feb 5. Erratum in: J Clin Endocrinol Metab. 2021 Jun 16;106(7):e2845. PMID: 18252793.
Martin KA, Anderson RR, Chang RJ, Ehrmann DA, Lobo RA, Murad MH, Pugeat MM, Rosenfield RL. Evaluation and Treatment of Hirsutism in Premenopausal Women: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018 Apr 1;103(4):1233-1257. doi: 10.1210/jc.2018-00241. PMID: 29522147.
Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr Rev. 2016 Oct;37(5):467-520. doi: 10.1210/er.2015-1104. Epub 2016 Jul 26. PMID: 27459230; PMCID: PMC5045492.
Kindly Let Us Know If This Was helpful? Thank You!


