Reproductive Endocrinology
8 Topics Diabetes Mellitus
24 Topics Calcium and Bone Metabolism
7 Topics Pituitary Disorders
2 Topics Lipidology and Obesity
13 Topics Thyroid Diseases
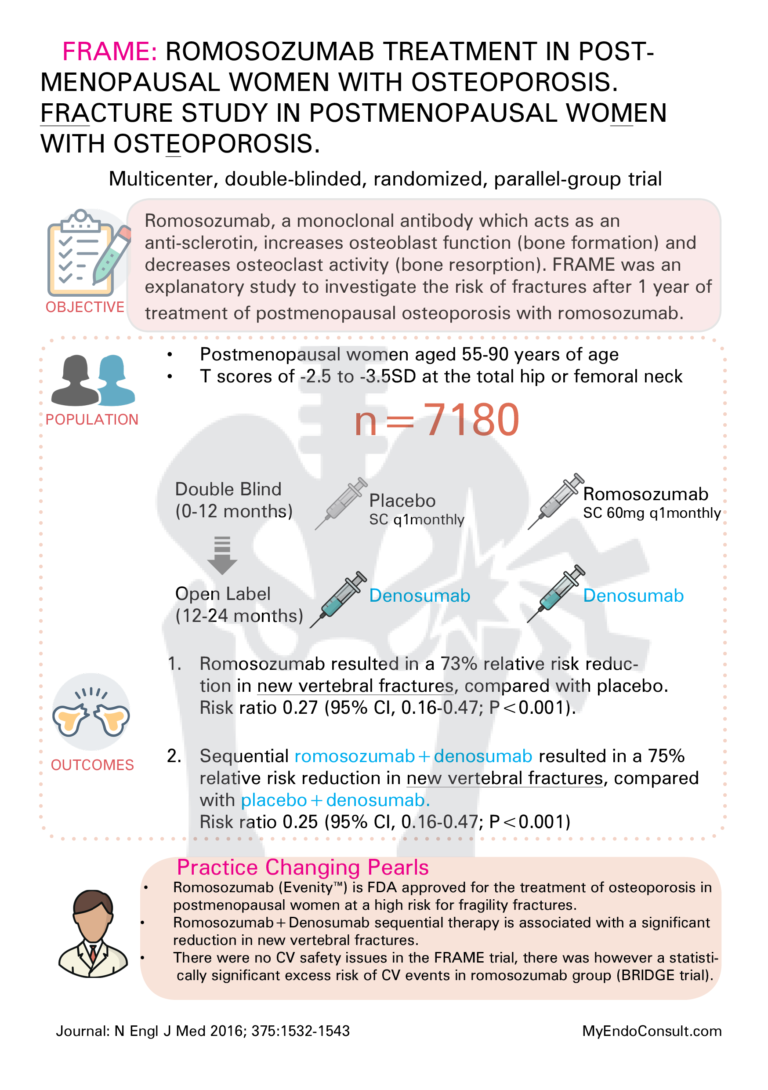
7 Topics FRAME : Romosozumab Treatment in Postmenopausal Women with Osteoporosis
FRAME : Romosozumab Treatment in Postmenopausal Women with Osteoporosis
• Romosozumab (Evenity™) is FDA approved for the treatment of osteoporosis in postmenopausal women at high risk for fragility fractures.
• Romosozumab+Denosumab sequential therapy is associated with a significant reduction in new vertebral fractures.
• There were no CV safety issues in the FRAME trial, there was however a statistically significant excess risk of CV events in romosozumab group (BRIDGE trial).
Infographic
References
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CA, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med. 2016 Oct 20;375(16):1532-1543.

