Orforglipron vs. Type 2 Diabetes: A Breakthrough in Oral Treatment?
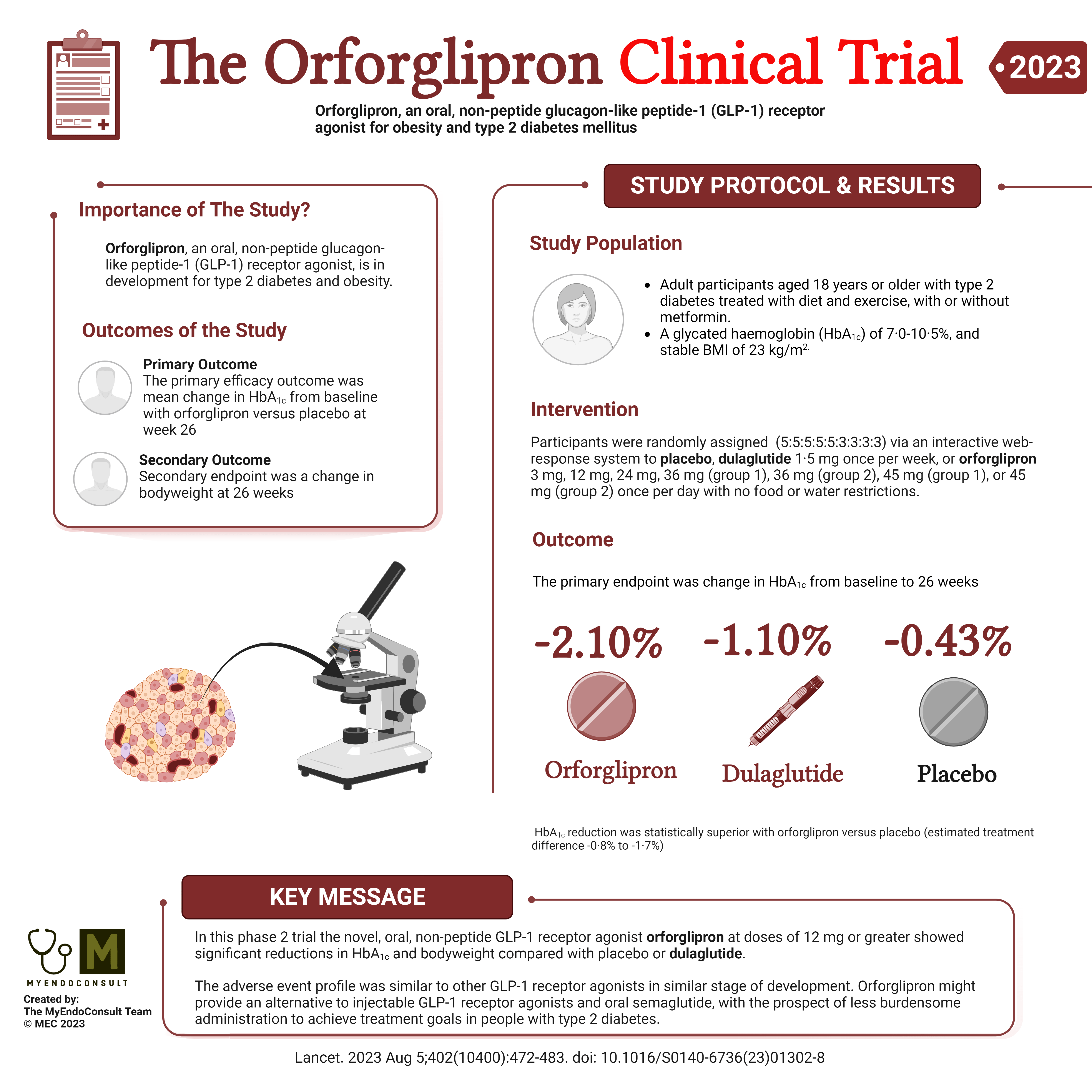
Study Synopsis:
🔍 Objective: Orforglipron, an exciting oral candidate in the quest against type 2 diabetes, has been put to the test! This non-peptide GLP-1 receptor agonist could change the game by offering an alternative to injections and other oral treatments.
🏥 Design: A 26-week, phase 2, randomized, double-blind study took place across multiple sites (USA, Hungary, Poland, Slovakia) where adult type 2 diabetes patients, on diet and exercise with/without metformin, were assessed for the efficacy and safety of orforglipron.
💊 Treatment Groups: Participants were given a variety of treatment options including orforglipron at different doses, dulaglutide (a known treatment), or placebo. There were no food or water restrictions.
📋 Key Outcomes:
- HbA1c Reduction: Orforglipron boasted a significant reduction in HbA1c, a key marker of diabetes control, surpassing both placebo and dulaglutide.
- Weight Change: Participants on orforglipron lost up to a remarkable 10.1 kg after 26 weeks, more than those on placebo and dulaglutide.
- Safety: While most reported side effects were of a gastrointestinal nature and were mild to moderate, orforglipron’s safety profile was consistent with other GLP-1 receptor agonists. A note of caution: some participants had hypoglycaemia, though it was clinically significant in only a few.
💡 Bottom Line: This study shines a hopeful light on orforglipron as a potential oral, non-peptide GLP-1 receptor agonist for type 2 diabetes treatment. The results suggest its superiority in improving glycemic control and promoting weight loss compared to existing treatments, but as always, further research is warranted.
🔗 Reference: ClinicalTrials.gov (NCT05048719).
Sponsor’s Note: A big shout-out to Eli Lilly and Company for funding this insightful research!

