iDCL: The international Diabetes Closed Loop Trial: The Clinical Acceptance of the Artificial Pancreas
iDCL: The international Diabetes Closed-Loop Trial: The Clinical Acceptance of the Artificial Pancreas
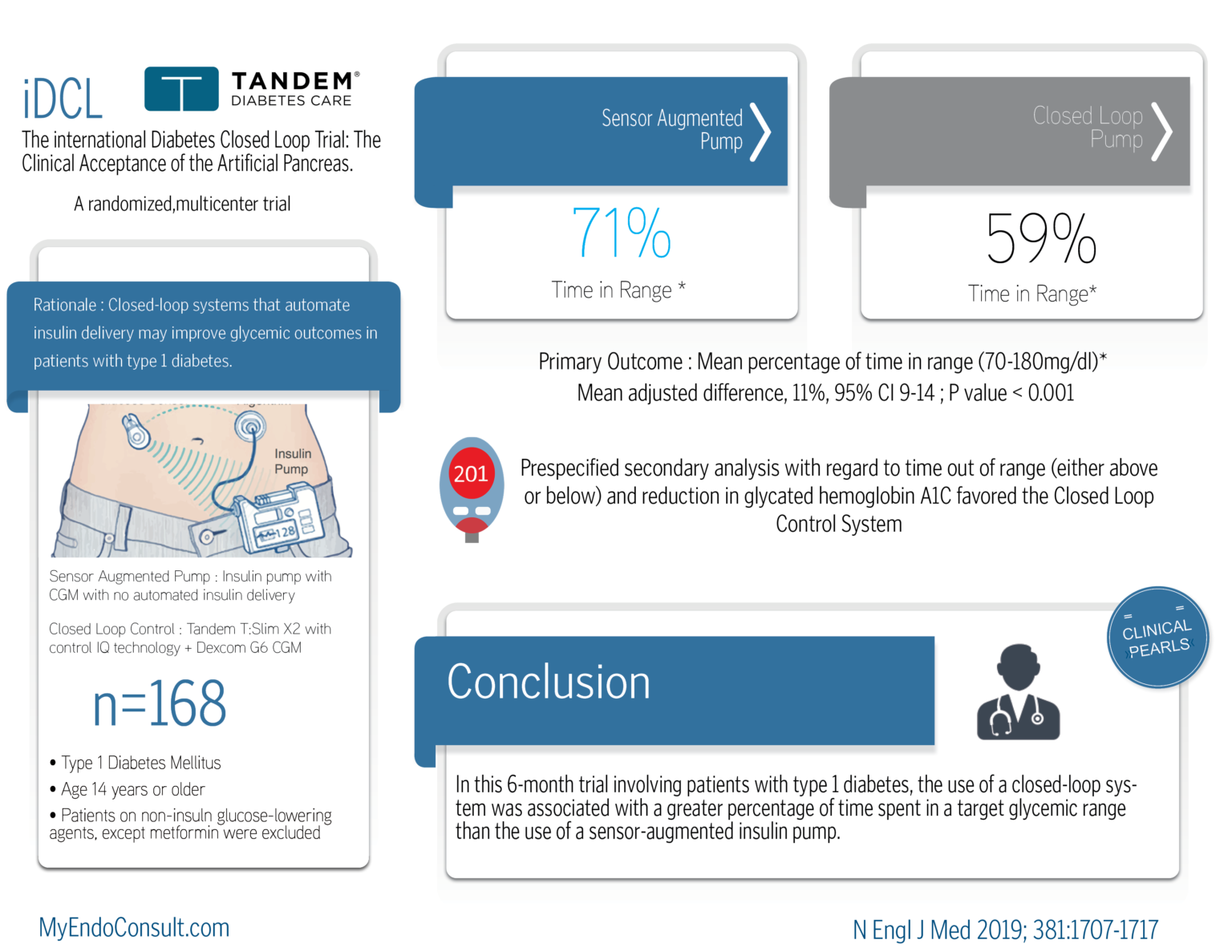
Sensor Augmented Pump: Insulin pump with CGM with no automated insulin delivery
Closed-Loop Control: Tandem T Slim X2 with control IQ technology + Dexcom G6 CGM
Prespecified secondary analysis with regard to time out of range (either above or below) and reduction in glycated hemoglobin A1C favored the Closed Loop Control System.
In this 6-month trial involving patients with type 1 diabetes, the use of a closed-loop system was associated with a greater percentage of time spent in a target glycemic range than the use of a sensor-augmented insulin pump.
Infographic
Reference
Anderson SM, Dassau E, Raghinaru D, Lum J, Brown SA, Pinsker JE, Church MM, Levy C, Lam D, Kudva YC, Buckingham B, Forlenza GP, Wadwa RP, Laffel L, Doyle FJ 3rd, DeVries JH, Renard E, Cobelli C, Boscari F, Del Favero S, Kovatchev BP. The International Diabetes Closed-Loop Study: Testing Artificial Pancreas Component Interoperability. Diabetes Technol Ther. 2019 Feb;21(2):73-80.

