Reproductive Endocrinology
8 Topics Diabetes Mellitus
24 Topics Calcium and Bone Metabolism
7 Topics Pituitary Disorders
2 Topics Lipidology and Obesity
13 Topics Thyroid Diseases
7 Topics A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis
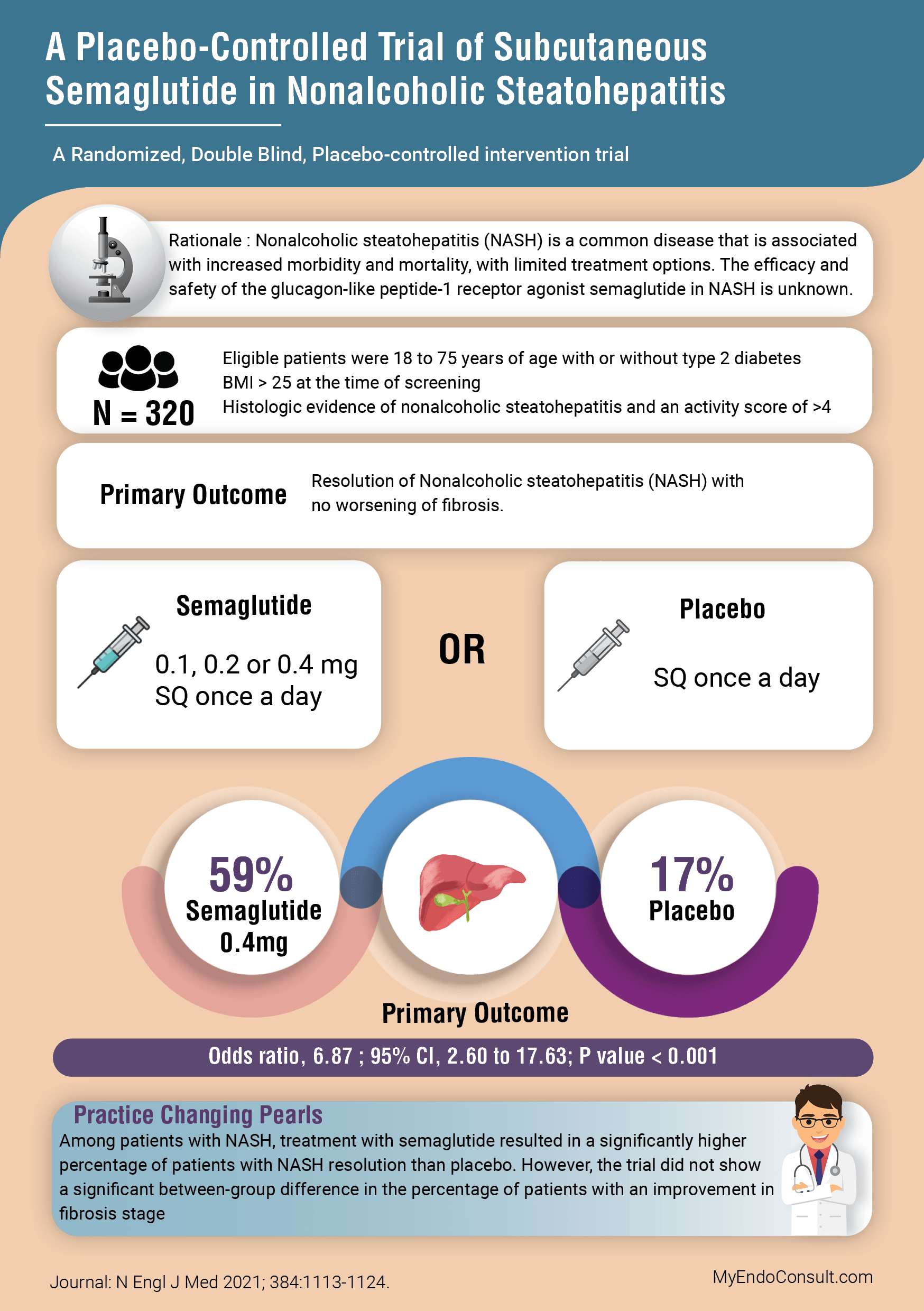
A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis
Rationale : Nonalcoholic steatohepatitis (NASH) is a common disease that is associated
with increased morbidity and mortality, with limited treatment options. The efficacy and
safety of the glucagon-like peptide-1 receptor agonist semaglutide in NASH is unknown.
Infographic
Reference
Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 2021 Mar 25;384(12):1113-1124.

