FREEDOM : Denosumab for prevention of fractures in postmenopausal women with osteoporosis
FREEDOM : Denosumab for prevention of fractures in postmenopausal women with osteoporosis
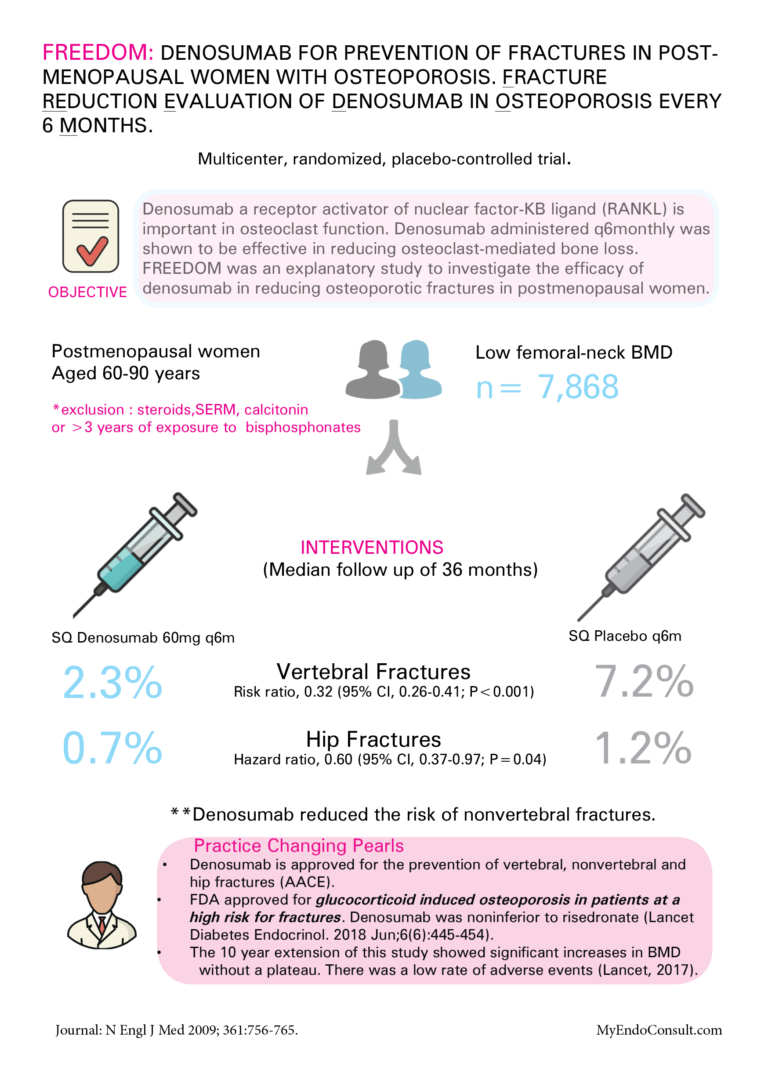
• Denosumab is approved for the prevention of vertebral, nonvertebral, and hip fractures (AACE).
• FDA approved for glucocorticoid-induced osteoporosis in patients at high risk for fractures. Denosumab was non-inferior to risedronate (Lancet Diabetes Endocrinol. 2018 Jun;6(6):445-454).
• The 10-year extension of this study showed significant increases in BMD without a plateau. There was a low rate of adverse events (Lancet, 2017)
Infographics
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009 Aug 20;361(8):756-65. doi: 10.1056/NEJMoa0809493. Epub 2009 Aug 11. Erratum in: N Engl J Med. 2009 Nov 5;361(19):1914.

