A concise review of adrenal gland physiology and classic adrenal gland diseases (adrenal insufficiency, Cushing’s syndrome, Pheochromocytoma/Paraganglioma, Adrenal Incidentaloma, and Primary Hyperaldosteronism). A summary of practical clinical pearls of adrenal gland pathology.
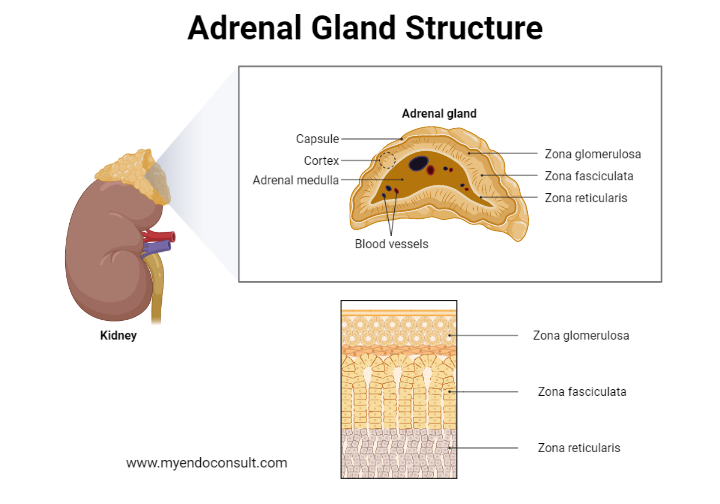
Adrenal Physiology
Aldosterone (Secreted by the Zona Glomerulosa)
- Action: ↑ Na reabsorption, ↑ K excretion, inflammation, and fibrotic effect on blood vessels/heart. It is under the control of the Renin-angiotensin-Aldosterone system
- Stimulus: Hypovolemia, hypotension, hyperkalemia
- Excess: Primary hyperaldosteronism = HTN and hypokalemia
- Deficiency: Primary adrenal insufficiency (AI)
Cortisol (Secreted by the Zona Fasciculata)
- Action: metabolism, vascular, immune effects. Controlled under pituitary-adrenal axis
- Stimulus: Stress (hypotension, hypoglycemia, etc.) – through ACTH action
- Excess: Cushing’s syndrome
- Deficiency: Primary and secondary AI
Dehydroepiandrosterone (DHEA) (Secreted by the Zona Reticularis)
- Action: weak androgen, converted to Testosterone peripherally, source of androgens in females, trivial significance in males
- Stimulus: ACTH
- Deficiency: Questionable endocrine significance
Catecholamine (Epinephrine and norepinephrine) (Secreted by the medulla)
- Action: sympathetic stimulation through adrenergic receptors (increased heart rate, blood pressure, metabolic rate)
- Stimulus: hypotension, physical/psychological stress, hypoglycemia
- Excess: Pheochromocytoma
- Deficiency: Questionable endocrine significance
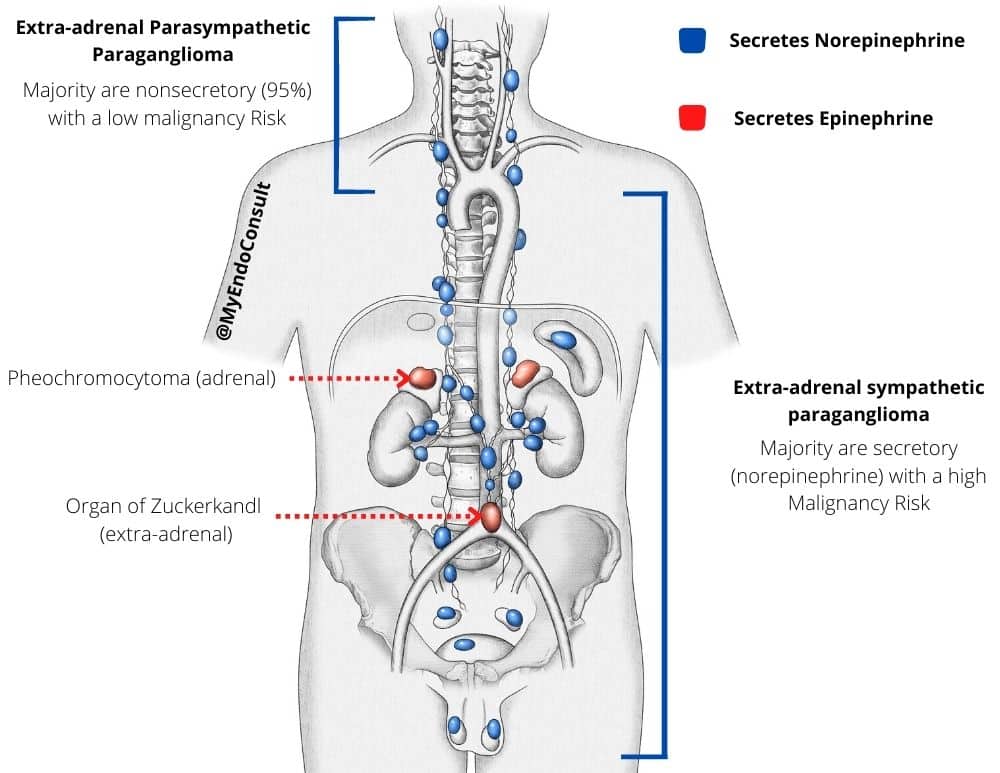
Pheochromocytoma and Paraganglioma (PPGL)
PPGL refers to a catecholamine secreting tumor arising from chromaffin cells. Classification of these tumors is dependent on their origin.
- Adrenal medulla = Pheochromocytoma
- Sympathetic Paraganglia = Paraganglioma
It is worthy to note that a third of PPGLs are associated with a germline mutation. Genetic counseling/referral should be considered in all patients presenting with PPGLs.
- NF1
- MEN2
- VHL
- Succinate dehydrogenase (SDH) gene mutation
Clinical Presentation of PPGL
Hypertension may be episodic or persistent; however, some patients may have normal blood pressure
- Hyperadrenergic spells (found in less than 50% of patients)
- Palpitation, sweating, headache
- Pallor, abdominal pain, acute MI/stroke
The severity of symptoms is well correlated with catecholamine level. Patients with triad symptoms from PPGL have catecholamine at least 4 times the upper normal limits and always have visible adrenal/paraganglioma on imaging.
When to suspect PPGL?
- Resistant hypertension
- Early onset hypertension < 20 years old
- Hyperadrenagic spell
- Familial syndrome or family history of pheochromocytoma
- Adrenal incidentaloma
- Idiopathic cardiomyopathy
Diagnostic Approach
Biochemical diagnosis
Both have a high sensitivity. Plasma has slightly less specificity than urine metanephrines. In patients with a low clinical suspicion (low pretest probability for PPGL), the urine test is preferred to reduce the risk of false positives.
Imaging for localization
– CT/MRI abdomen
– I-123 MIBG scan for high-risk tumor (large tumor > 10 cm is concerning malignant pheochromocytoma and metastasis)
Measurement of Catecholamine metabolites
- Epinephrine = metanephrine (MN)
- Norepinephrine = normetanephrine (NMN)
- Total metanephrines = MN + NMN
- Total metanephrines ≥ 4 times the upper limit of normal (ULN) strongly suggests the diagnosis
Certain medications can cause falsely high metanephrines
- TCA
- Cyclobezaprine (Flexeril)
- Taper or DC these medications for at least two weeks before retesting
Treatment
- Surgical resection: Adrenalectomy
- A catecholamine crisis can occur during the surgery
Pre-op preparation is a must
- Alpha blockage FIRST titrate until orthostatic hypotension and tachycardia develop
Irreversible alpha-blocker: Phenoxybenzamine ($$$)
Reversible alpha-blocker: Doxazosin ($)
- Beta blockage AFTER alpha blockage is adequate (orthostatic hypotension and tachycardia develop)
- To treat reflex tachycardia
- Adjunct: CCB, Metyrosine if blood pressure remains uncontrolled(e.g., patients with a large tumor).
- Aldosterone excess
- Common cause of secondary hypertension
- Hypokalemia (either spontaneous or diuretic-induced) may not be presented in half of the patients with PA
Etiology
- Bilateral adrenal hyperplasia (2/3)
- Adrenal adenoma (1/3) – tumor may be very small and not well seen from the CT
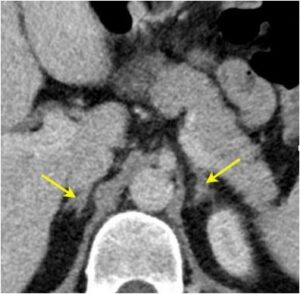
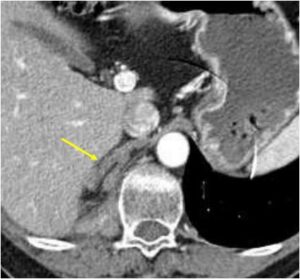
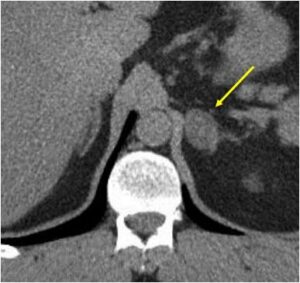
| Computed Tomography Findings | Description |
| Normal Adrenal Gland
| Reverse “Mercedes Benz Sign” A limb of the adrenal gland is no greater than the crus of the diaphragm. |
| Adrenal hyperplasia
| Medial and lateral limbs of the adrenal gland are larger than the width of the diaghramatic crus. |
| Adrenal adenoma
| Diffuse swelling of the adrenal gland. Blunting of the medial and lateral limbs in this image. |
When should a primary care physician suspect primary hyperaldosteronism?
- Untreated HTN with sustained BP > 150/100
- Resistant HTN
- HTN with adrenal incidentaloma
- HTN with spontaneous or diuretic-induced hypokalemia
- HTN with FH of PA in 1st-degree relative or FH of HTN diagnosed at < Age 40
Diagnostic Approach
1) Screening test: Plasma aldosterone/renin ratio (ARR)
Positive test: ARR 20 AND plasma aldosterone > 15 ng/dL. Refer to endocrinology if the screening test is positive
Pathophysiology Concept: High aldosterone will suppress renin activity in normal conditions. Thus a low renin activity with high aldosterone suggests PA.
Blood pressure medications can cause a falsely negative or positive ARR result
- You can check ARR while patients are on most of their BP meds (EXCEPT Aldosterone antagonist)
- ACEI/ARB will increase renin level
- Suppressed renin level while on these medications strongly suggests PA
- Non-suppressed renin level makes PA unlikely
- If equivocal result, then replace with a medication that interferes less with ARR (verapamil, Alpha blocker) and repeat ARR in 2 weeks.
- Aldosterone antagonist will always increase renin activity level (Stop for six weeks before retesting ARR).
2) Confirmatory test
- 24 hr urine sodium and urine aldosterone, Fludrocortisone Suppression test, Saline infusion or salt loading test.
- May not need if the screening test is very suggestive of PA
3) Localization
- CT abdomen
- Adrenal vein sampling to localize the source of aldosterone excess
Treatment
Surgical treatment
- Resolution of HTN in 40% of the patients
- Transient hypoaldosteronism (hyperkalemia, low BP) after surgery may occur
Medical treatment
- When a patient is not a surgical candidate
- Aldosterone antagonist
- Spironolactone ($): side effects – gynecomastia, irregular periods in women
- Eplerenone ($$$)
Cushing Syndrome
Cortisol regulation
- Increased cortisol production, resulting in stigmata of hypercortisolism.
- Lack of diurnal cortisol rhythm
- Failure to adequately suppress cortisol levels after dexamethasone administration
The causes of hypercortisolism
- Pituitary Cushing disease
- Ectopic Cushing syndrome
- Adrenal Cushing syndrome
- Exogenous Steroid
Clinical findings
Direct Cortisol effect
- Fat redistribution “Cushingoid features.”
- Weight gain
- Hyperglycemia
- Osteoporosis
- Thinning of skin (Violaceous striae > 1 cm, “cigarette paper skin” or Liddle’s sign)
- Proximal muscle weakness
Mineralocorticoid effect: seen in more severe hypercortisolism
- Hypokalemia with metabolic alkalosis
- Androgen effect: Hirsutism (due to DHEAS co-secretion)
What is Pseudo-Cushing’s syndrome
- It can be challenging to differentiate from true Cushing’s syndrome
- Similarly, patients with pseudo-Cushing’s lose diurnal cortisol rhythm and fail to suppress cortisol levels after dexamethasone administration.
- Abdominal obesity, hypertension, hyperlipidemia, and insulin-resistance
- Common causes of PC include patients with the following conditions major depression, alcoholism, and uncontrolled diabetes
Diagnostic Approach
- Confirm hypercortisolism state with biochemical test: blood, urine, salivary
- ACTH-independent vs. ACTH-dependent hypercortisolism
- Localize the source of hypercortisolism with imaging. Read this recent article on hypercortisolemia in the setting of bilateral adrenal lesions.
Treatment
- Surgery: removing the source of hypercortisolism
- Medical: Medical management of Cushing’s syndrome
Always initiate steroid (hydrocortisone) replacement after surgery for Cushing’s of any underlying etiology. Adrenal insufficiency will always occur after an adrenalectomy.
Adrenal insufficiency
Adrenal insufficiency (AI)
Primary (Adrenal gland)
- Loss of ALL adrenal hormones
- High ACTH – hyperpigmentation
- Hypotension, HypoNa, HyperK, Hypoglycemia
- Imaging: Atrophic or enlarged adrenal glands (depends on the cause of primary AI). Calcification of the adrenal gland may be seen in some patients (post-inflammatory or infective changes)
Secondary (Pituitary or hypothalamic)
- Spares Aldosterone production
- Low or low normal ACTH with no hyperpigmentation
- Hypotension (due to lack of permissive effect on catecholamine), hyponatremia (inappropriate increase in vasopressin secretion/action due to cortisol deficiency), and hypoglycemia.
- Hyperkalemia is rare as aldosterone is intact.
- Imaging: Atrophic adrenal glands if chronic
Causes of primary adrenal insufficiency
- Autoimmune (Addison’s disease)
- Most common cause of primary AI in the US
- 90% have positive anti-21 hydroxylase antibody
- Associated with other autoimmune diseases (Hashimoto’s thyroiditis, T1DM, Celiac disease, autoimmune hypoparathyroidism etc.). A part of the autoimmune polyglandular syndrome
- Infiltrative disease involving both adrenal glands (Tuberculosis /Fungal infections/sarcoidosis/lymphoma)
- Rarely metastatic cancer to adrenal glands will cause AI
- Bilateral adrenal hemorrhage
- Coagulopathy/anti-coagulant
Treatment of primary AI
- Aldosterone replacement: Fludrocortisone
- Cortisol replacement:
- Hydrocortisone 15-20 mg a day, divided into 2-3 times a day with a higher dose in AM
- Prednisone 5 mg/Methylprednisone 4 mg/Dexamethasone 0.75 mg a day (less preferred)
- Avoid over-replacement –> iatrogenic Cushing
- DHEA replacement– controversial, may provide improvement in QOL in some female patients
Sick day rules
- Minor illness or minor surgery
- Double or Triple the dose of glucocorticoid for three days (or until feeling better)
- Major stress or surgery
- Hydrocortisone 100 mg IV q 8 hrs and taper down once clinically improving
“Relatively adrenal insufficiency”
A controversial term used to explain refractory hypotension in ICU patients without a history of AI. It is based on the theory that these patients are not able to produce enough cortisol as a stress response
It is hard to prove due to various reasons:
- Low cortisol-binding globulin, interference with cortisol assay by various medication effects, etc.
- No agreement on diagnostic criteria
- No improved survival in pt with “relative AI” treated with stress dose steroid. However, an improvement in blood pressure after stress dose steroid administration has been demonstrated in clinical trials.
- Current Consensus Recommendation: Consider Stress doses of hydrocortisone in patients with shock that are resistant/refractory to fluid and vasopressor administration.
Adrenal incidentaloma
Adrenal mass > 1cm in diameter that is detected incidentally on imaging for other reasons
Two critical endocrine questions
- Is this cancer? (Measurement of Hounsfield units on non-contrast computed tomography)
- Is this functioning (producing any hormone) : Aldosterone (primary hyperaldosteronism), Cortisol (Cushing’s syndrome) and Epinephrine/normetanephrine (Pheochromocytoma)
An adrenalectomy is recommended if the answer is “yes” to any of the questions.
Do not biopsy an adrenal mass!!!
Possible contraindications to an adrenal biopsy
Adrenal biopsy cannot differentiate between benign adrenal adenoma
and adrenal cancer. Furthermore, seeding of the needle track by adrenal cancer is possible and finally, if the mass is indeed a pheochromocytoma, biopsy can cause a catecholamine crisis!
In rare circumstances, an adrenal biopsy can be considered if there is a suspicion of adrenal infiltrative disease (metastatic cancer to adrenal glands, fungal infection, etc). However, always rule out a pheochromocytoma before a biopsy.
Confirming Functionality.
- Rule out Cushing syndrome: 1 mg Dexamethasone suppression test
- Rule out Pheochromocytoma (unless the adrenal mass was read as lipid-rich adenoma or Hounsfield unit < 10).
- Rule out primary hyperaldosteronism: Plasma aldosterone/renin activity in ALL patients with hypertension.
References
- Grouzmann E, Drouard-Troalen L, Baudin E, Plouin P-F, Muller B, Grand D, Buclin T (2010) Diagnostic accuracy of free and total metanephrines in plasma and fractionated metanephrines in urine of patients with pheochromocytoma. Eur J Endocrinol 162:951–960
- Armanini D, Calò L, Semplicini A. Pseudohyperaldosteronism: pathogenetic mechanisms. Crit Rev Clin Lab Sci. 2003 Jun;40(3):295-335.
- John W. Funder, Robert M. Carey, Franco Mantero, M. Hassan Murad, Martin Reincke, Hirotaka Shibata, Michael Stowasser, William F. Young, Jr, The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 5, 1 May 2016, Pages 1889–1916, https://doi.org/10.1210/jc.2015-4061
- Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. 2004 Dec;136(6):1227-35.
- Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008 May;93(5):1526-40.
- Liddle GW. Tests of pituitary-adrenal suppressibility in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 1960 Dec;20:1539-60.
Kindly Let Us Know If This Was helpful? Thank You!